Method for preparing anti-tumor lead compound Tagalsin C by utilizing tagalsin E and Tagalsin G
A technology of lead compounds and compounds, applied in the field of pure chemistry, can solve the problems of complicated extraction process, lack of TagalsinC anticancer activity, and increased cost of TagalsinC drug research and development, and achieve the effect of simple process, high yield and few steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The present invention will be further described below in conjunction with specific examples, but the present invention is not limited to the following examples.
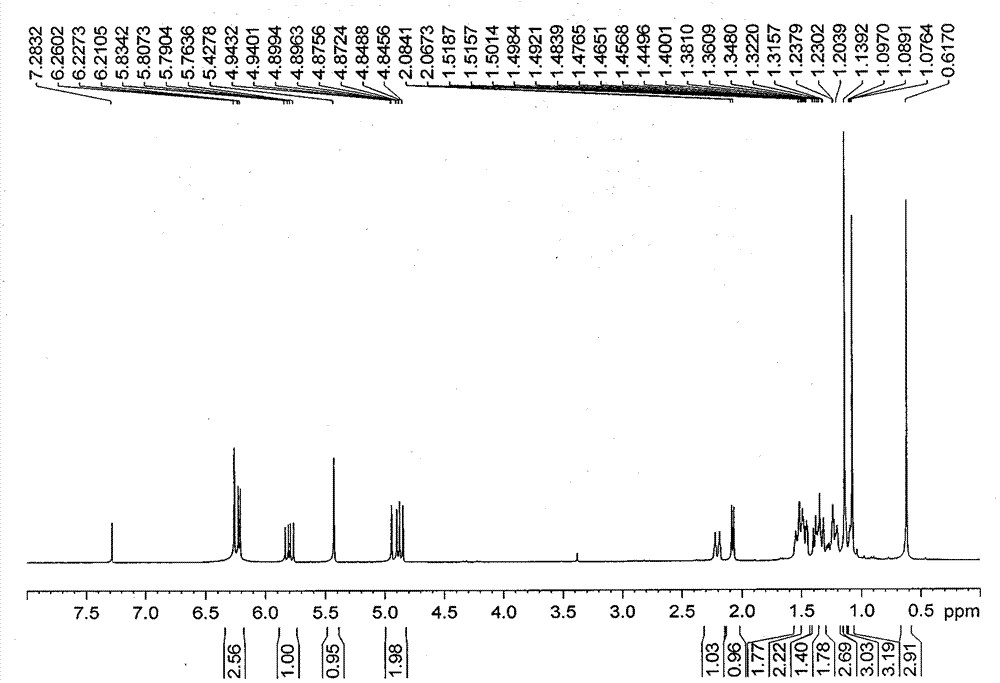
[0014] Take 0.1 mmol of Tagalsin E (28.6 mg) and 0.2 mmol of potassium tert-butoxide (22.4 mg) in a reaction tube, add 5 ml of anhydrous tetrahydrofuran solvent, and after the oil pump is vacuumed, feed oxygen, at -15 °C for 10 hours. The reaction was quenched with saturated ammonium chloride solution, and extracted with ethyl acetate to obtain an organic layer. The organic layer was concentrated under reduced pressure and purified by normal phase silica gel column chromatography with petroleum ether / ethyl acetate as the mobile phase to obtain 0.086 mmol of Tagalsin C.
[0015] Get 0.1 millimolar Tagalsin G (30.2 milligrams) and 0.1 millimoles palladium acetate (22.4 milligrams) in reaction tube, add the anhydrous dimethyl sulfoxide solvent of 5 milliliters, oil pump vacuumizes, and feeds oxygen, in The reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com