A method for rapid preparation of multiple anti-antibiotic hybridoma monoclonal antibodies
A technology of monoclonal antibodies and antibiotics, applied in chemical instruments and methods, hybrid peptides, animal/human proteins, etc., can solve problems such as antibiotic residues, difficulty in implementation, and difficulty in obtaining anti-antibiotic monoclonal antibody hybridoma cell lines , to achieve strong specificity, save time and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 antibiotic-carrier protein conjugate
[0026] For the cross-linking of antibiotics and KLH EDC CarrierProteinKits (ThermoScientific) was completed, and the specific operation was carried out according to the instructions. Briefly, 10 mg of KLH is dissolved in 1 mL of water to make a 10 mg per mL solution. 2 mg each of penicillin, streptomycin, tetracycline, oxytetracycline and gentamycin were dissolved in 2.25 ml of 0.1 MMES, 0.9 M NaCl, 0.02% sodiumazide, pH 4.7 buffer. Add KLH solution and antibiotic solution to a 15 ml centrifuge tube, mix well, then add 250 microliters of EDC with a concentration of 10 mg / ml to the reaction solution, mix well, and react at room temperature for 2 hours in the dark. Purify and isolate the antibiotic-KLH conjugate with the attached purification column.
Embodiment 2
[0027] Embodiment 2 Preparation of anti-antibiotic monoclonal antibody hybridoma cells
[0028] The prepared solution of complete Freund's adjuvant and antibiotic-carrier protein conjugate was mixed at a ratio of 1:1 to make an emulsion. Female Balb / c mice aged 6-8 weeks were immunized by subcutaneous multi-point injection (200 microliters per mouse, about 40 microliters per point). Two weeks later, the mice were boosted with multi-point subcutaneous injection of antibiotic-carrier protein conjugates in incomplete Freund's adjuvant. Two weeks later, blood was taken to determine the titer of antibodies. The mice were immunized by injecting antibiotic-carrier protein conjugates through the tail vein. Four days later, the mice were sacrificed, the spleen cells were isolated, and the isolated spleen cells were fused with mouse myeloma cells SP2 / 0 cells with 50% PEG. Then, selection culture was carried out in HAT-containing medium. Only confluent cells survive in HAT medium.
Embodiment 3
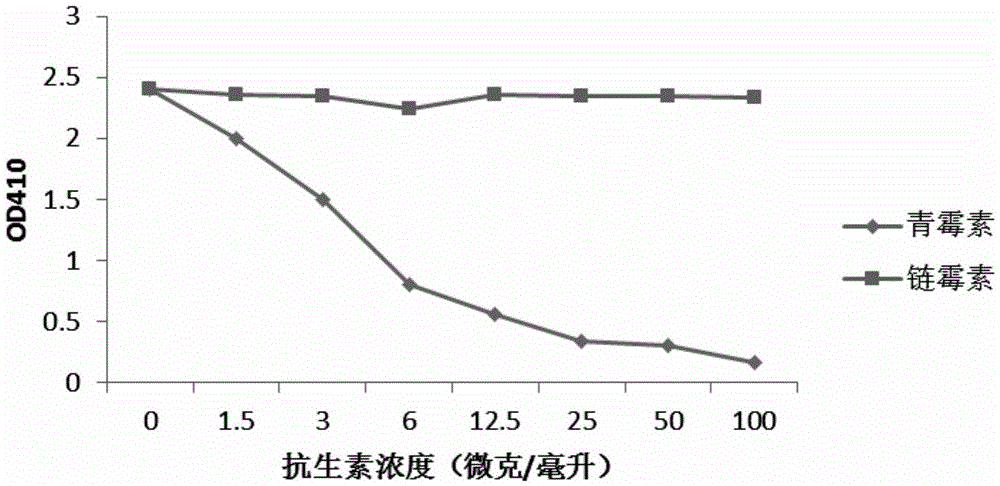
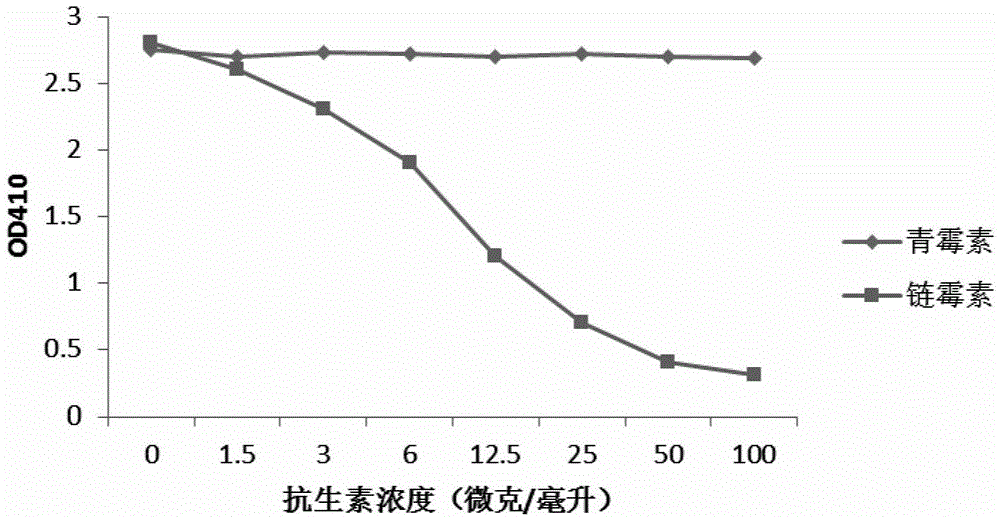
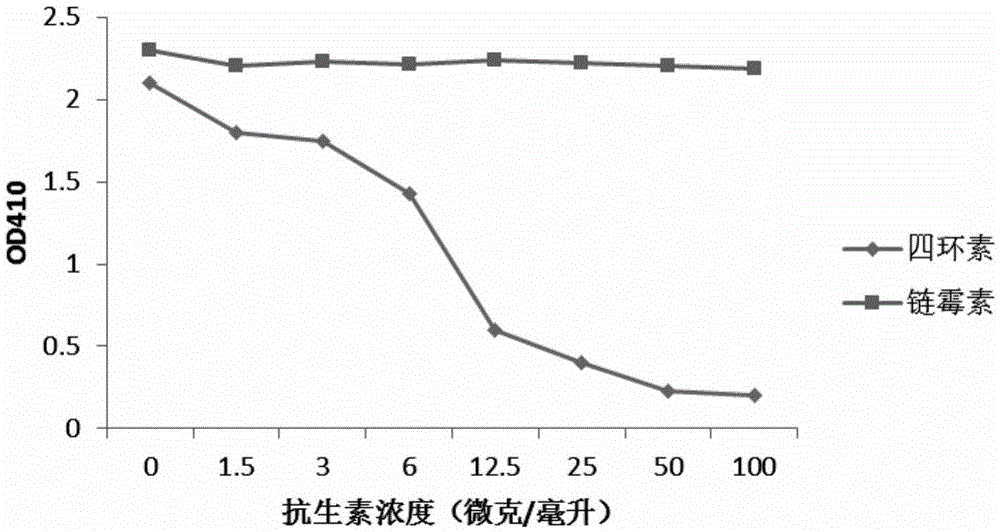
[0029] Example 3 Screening of Antibiotic Monoclonal Antibody Hybridoma Cells
[0030]Dissolve 100 microliters of antibiotic-bovine serum albumin conjugates (penicillin-bovine serum albumin, streptomycin-bovine serum albumin, tetracycline-bovine serum albumin, soil Mycin-Bovine Serum Albumin, Gentamicin-Bovine Serum Albumin, the above-mentioned antibiotic-Bovine Serum Albumin conjugates were purchased from SIGMA Company) were added to the small wells of the 96-well microtiter plate, and placed overnight at 4°C . On the second day, wash the antibiotic-bovine serum albumin-bound 96-well microtiter plate with washing solution (PBS buffer containing 0.05% Tween) twice. Then the microtiter plate was blocked with blocking solution (1%BSA / washing solution) at room temperature for two hours. The blocking solution was poured off, and then 100 microliters of the cell culture supernatant was added to a 96-well plate with the hybridoma culture solution obtained in Example 2, incubated at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com