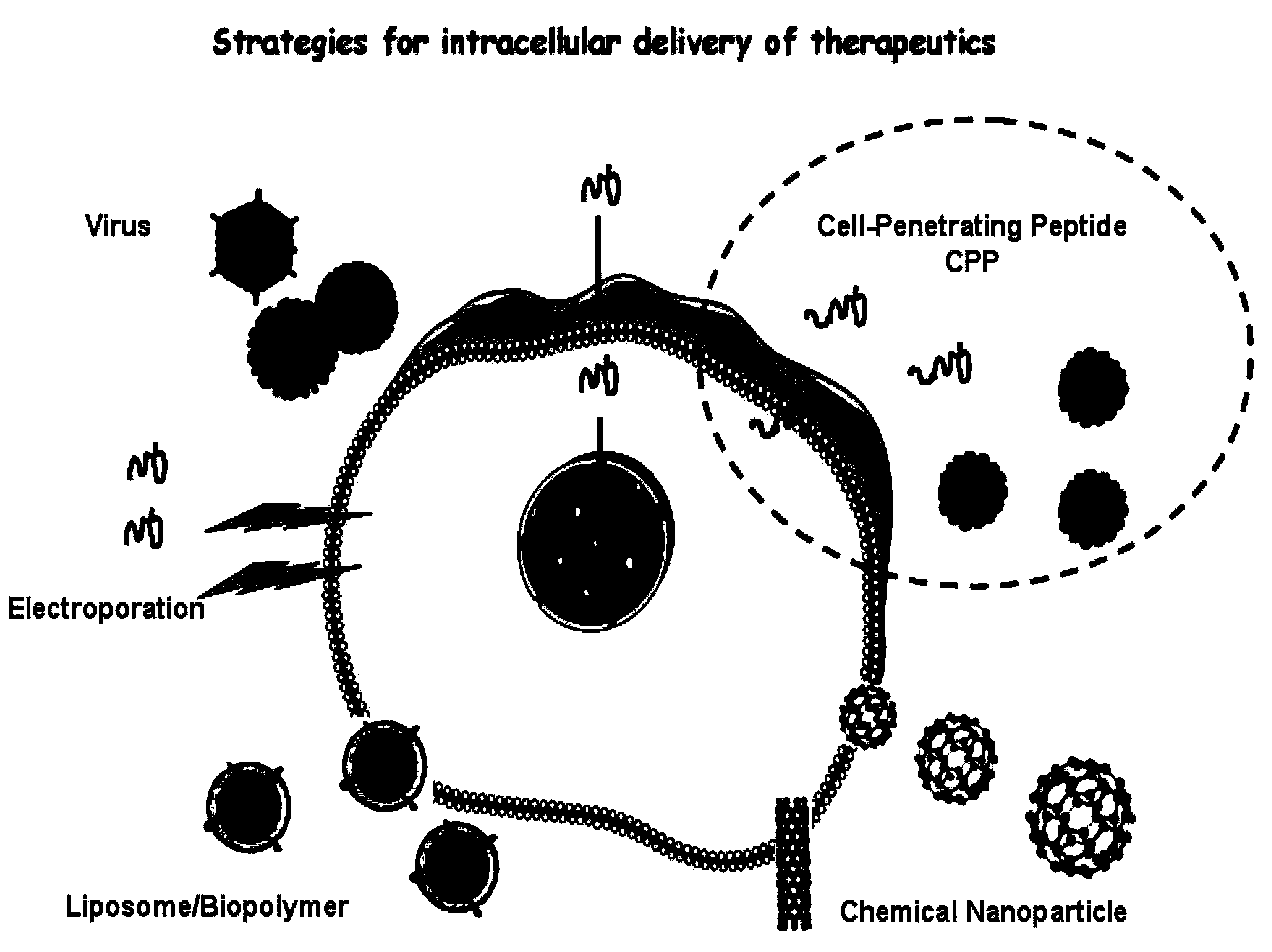
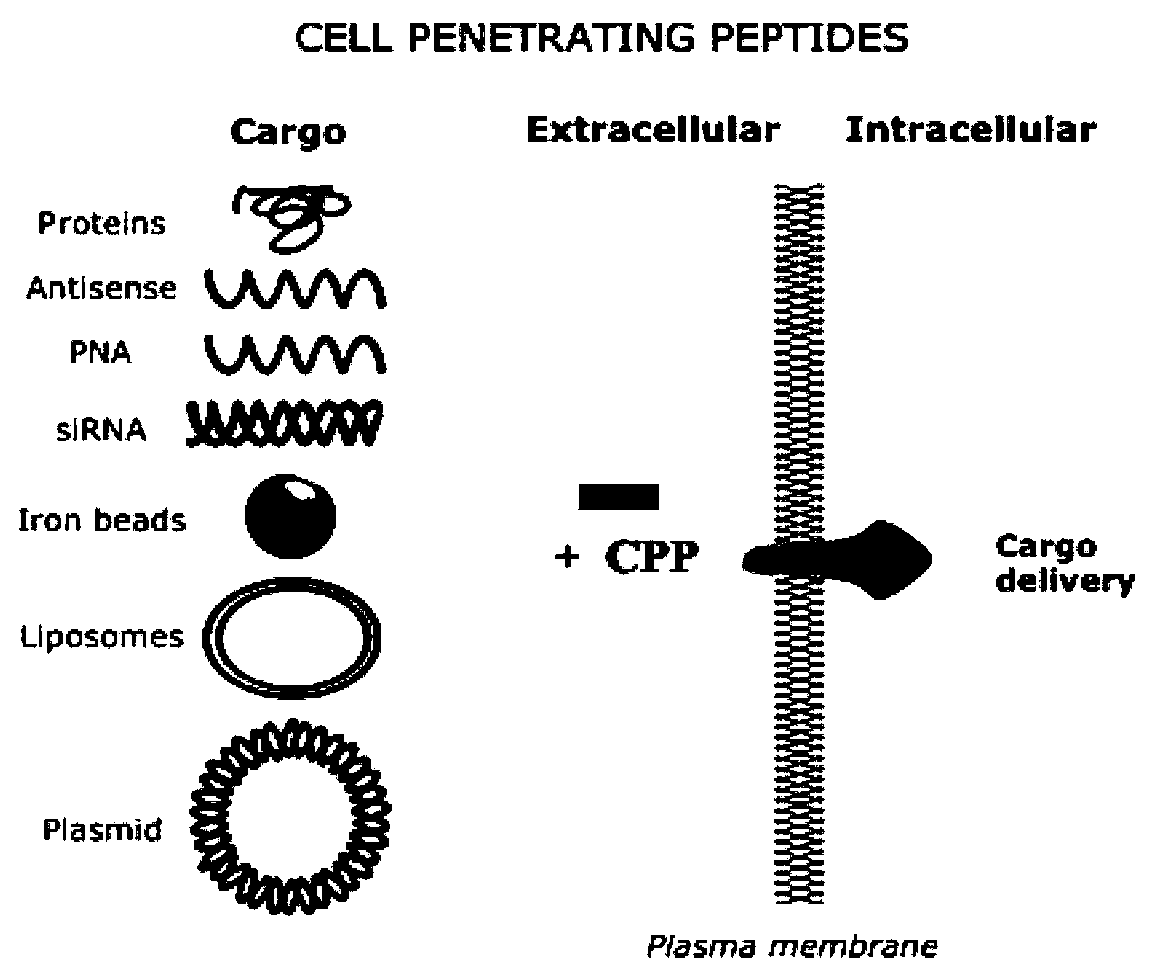
Cell permeable peptide hPP3 and usage thereof
A technology of penetrating peptides and cells, which is applied in the field of biomedicine to achieve the effect of small immune response, small possibility and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
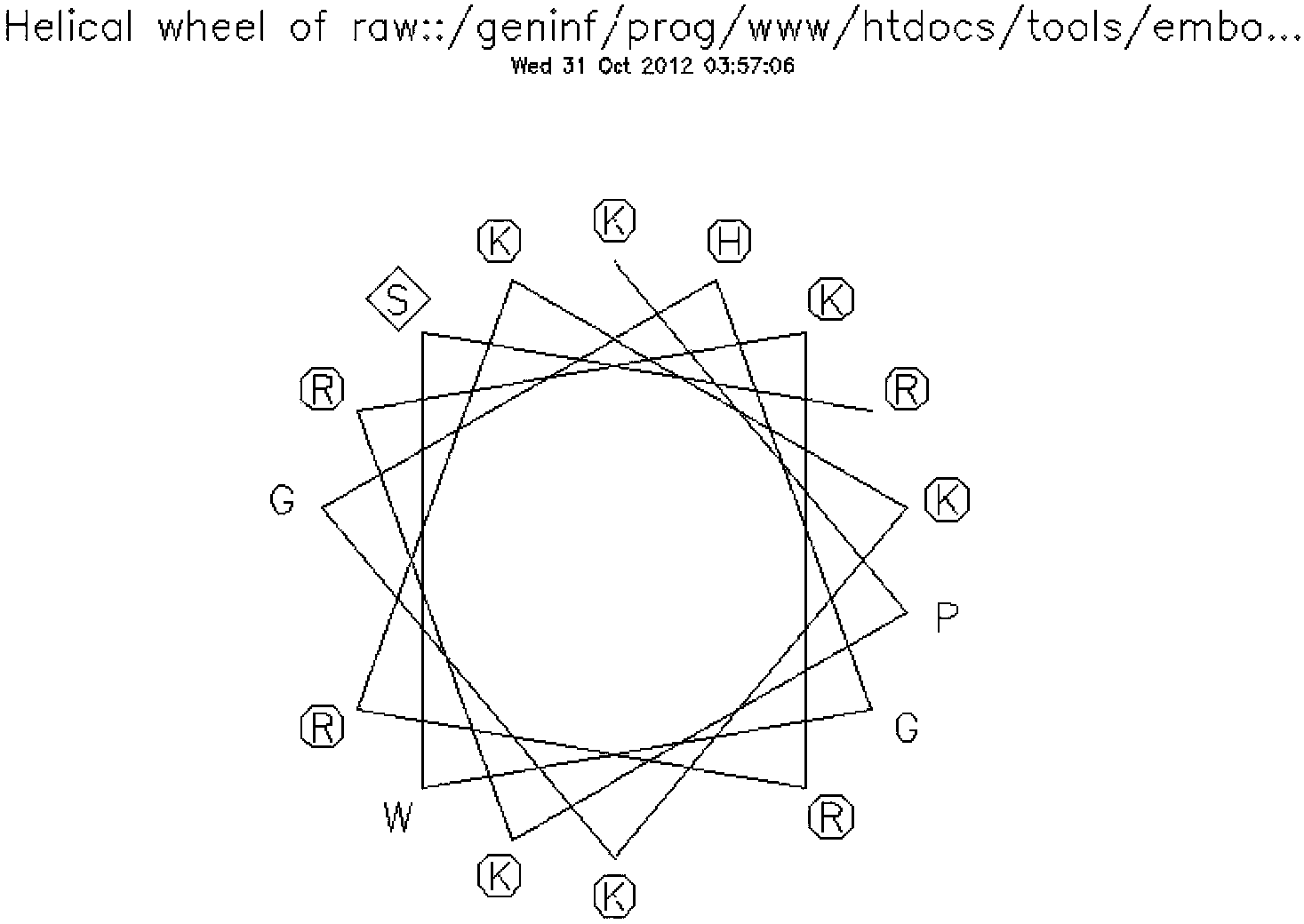
[0032] Example 1, CPP primary structure, secondary structure analysis, prediction and identification of new human-derived CPP:
[0033] 1. Analyze the secondary structure of the cell-penetrating peptide hPP3 obtained in the present invention, using the online analysis program of emboss (for the analysis program, please refer to the webpage: http: / / emboss.bioinformatics.nl / cgi-bin / emboss / pepwheel On-line analysis of the wheel structure of peptides; http: / / emboss.bioinformatics.nl / cgi-bin / emboss / help / garnier at Wire Analyze secondary structures (helices, folds, etc.). The schematic diagram of the wheel structure of hPP3, the schematic diagrams of the helical and folded structures are as follows image 3 and Figure 4 shown.
[0034] In order to facilitate the study of the cell transmembrane function of hPP3:
[0035] Chemically synthesized green fluorescently labeled hPP3:
[0036] Lys-Pro-Lys-Arg-Lys-Arg-Arg-Lys-Lys-Lys-Gly-His-Gly-Trp-Ser-Arg-FITC (hPP3-FITC);
[0037...
Embodiment 2
[0092] Example 2, hPP3 has little effect on cell viability
[0093] (1) Culture cells ECV-304 and HepG2 in the logarithmic growth phase at 1×10 4 The seeding density of each cell / well was inoculated in a 96-well plate for routine culture, 100 μl per well, and 3 replicate wells were set up for each cell, and cultured at 37°C;
[0094] (2) In the logarithmic growth phase, the culture medium was replaced with serum-free RPMI-1640 medium, and the culture was continued for 1 hour;
[0095] (3) Replace with serum-free RPMI-1640 culture medium with hPP3 concentration gradients of 10 μM, 20 μM, 30 μM, 40 μM and 50 μM, and continue to culture for 24 hours;
[0096] (4) After the incubation time is over, add 100 μl of PBS to each well to wash, 2 min×3 times;
[0097] (5) Add 80 μl serum-containing normal culture medium and 20 μl MTT (mother solution concentration 5 mg / ml, ie 0.5% MTT) solution to each well, continue to incubate at 37°C for 4 hours, and aspirate the culture medium af...
Embodiment 3
[0101] Example 3. Construction of pET15b-hPP3-GFP plasmid, expression and purification of fusion protein and research on its transmembrane efficacy
[0102] 3.1 Construction and identification of pET15b-hPP3-GFP recombinant plasmid
[0103] (1) Design two single-stranded cDNAs encoding hPP3, with Nde I and Xho I restriction sites on both sides, and submit them to Shanghai Shenggong Company to synthesize single-stranded oligonucleotide chains, and then combine the two single-stranded DNAs Add an equal amount into the aqueous solution, and cool naturally to room temperature at 95°C for 5 minutes to complete the annealing to form a complementary double-stranded DNA fragment (hPP3); at the same time, a pair of primers were designed using pEGFP (purchased from Clontech) as a template and obtained by PCR GFP protein gene fragment, with Xho I and BamH I restriction sites on both sides, purified PCR product for future use;
[0104] (2) Perform double digestion with BamH I / Nde I res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com