Testing method of in vitro relative efficiency of inactivated hepatitis A vaccine
A technology of inactivated vaccines and hepatitis A, applied in the biological field, can solve the problems of reducing testing costs and labor intensity, reducing vaccine inventory, and prolonging the validity period of vaccines, achieving reduced vaccine inventory, reliable testing methods, and good stability and reproducible effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
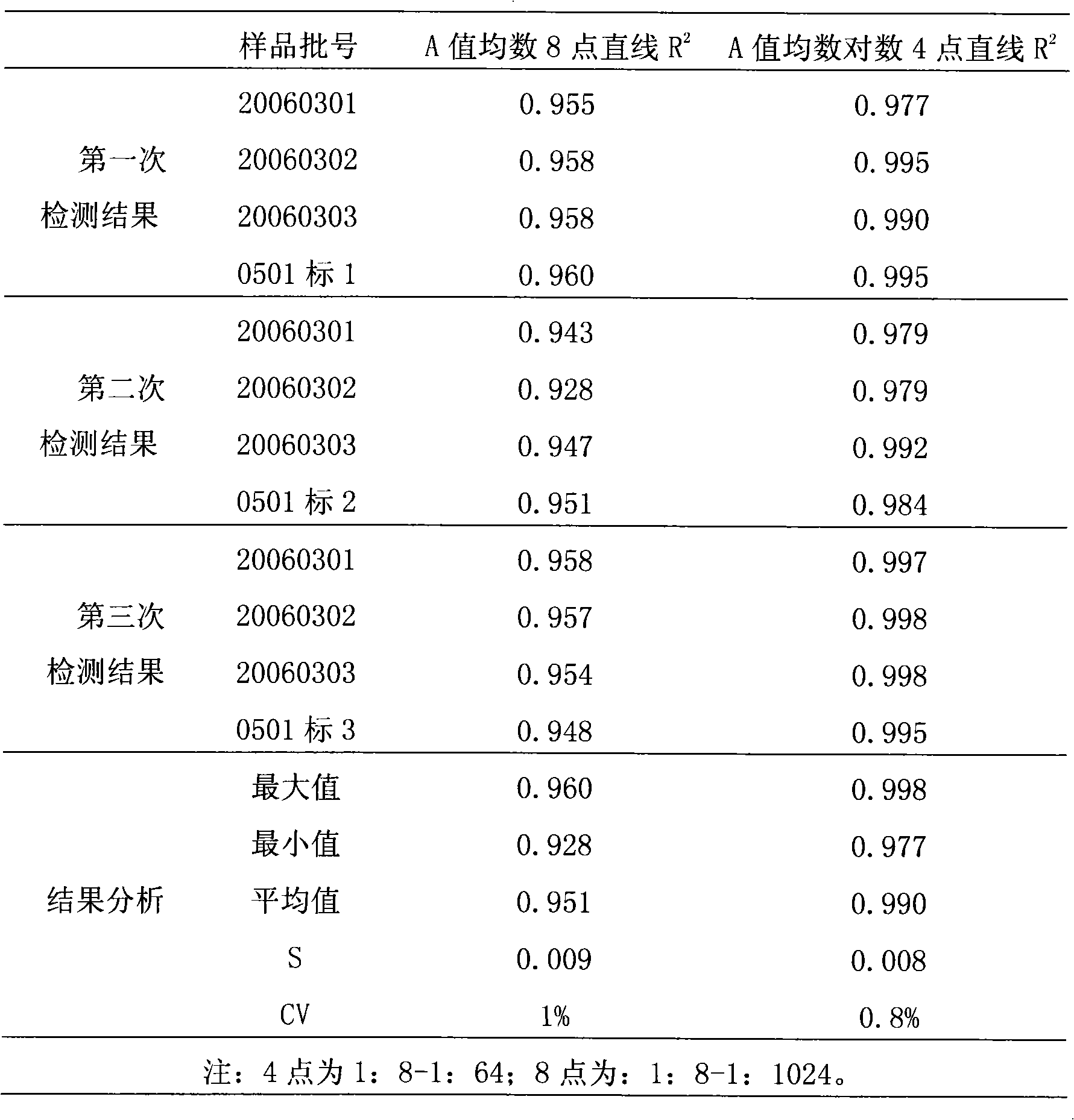
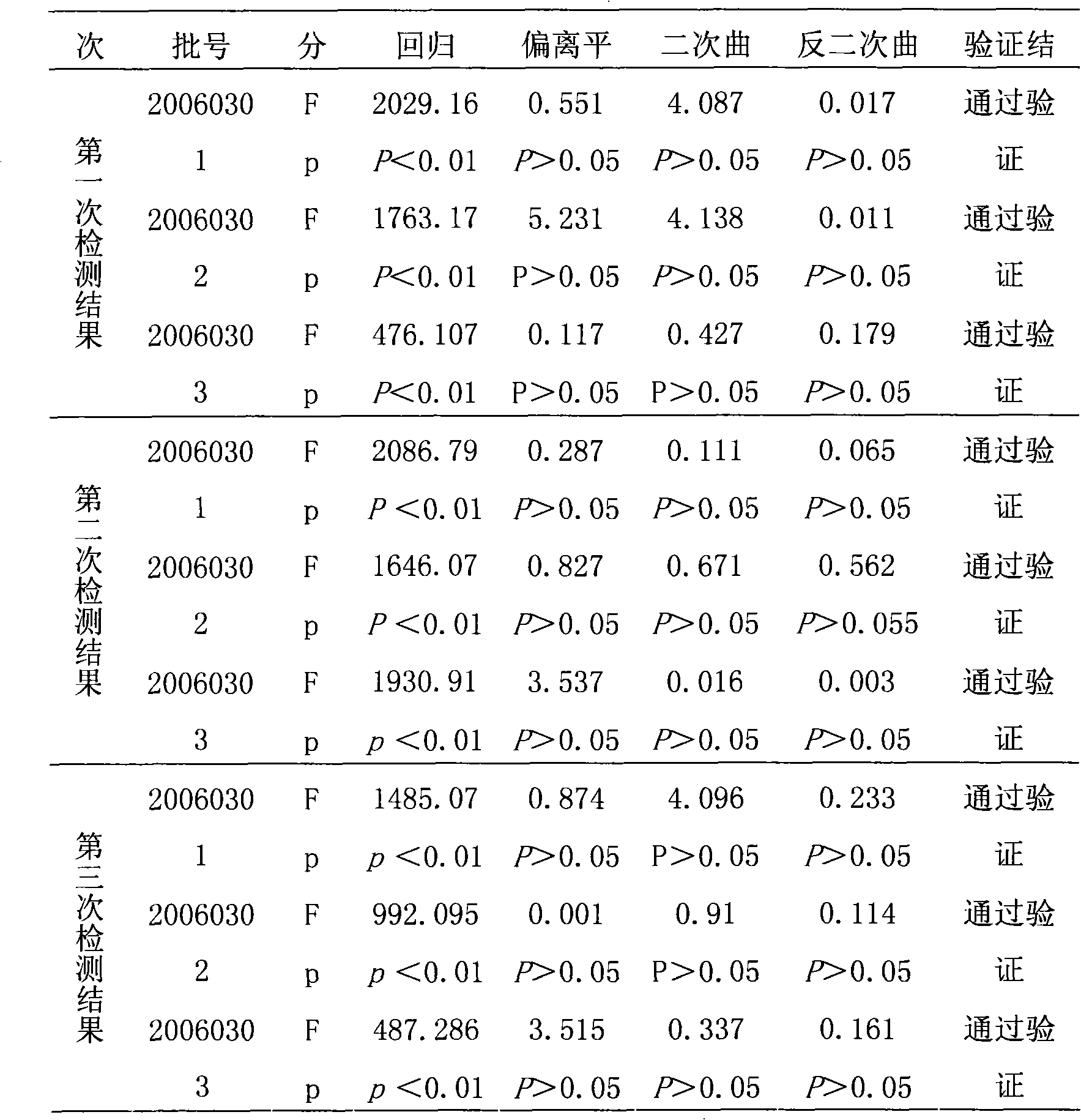
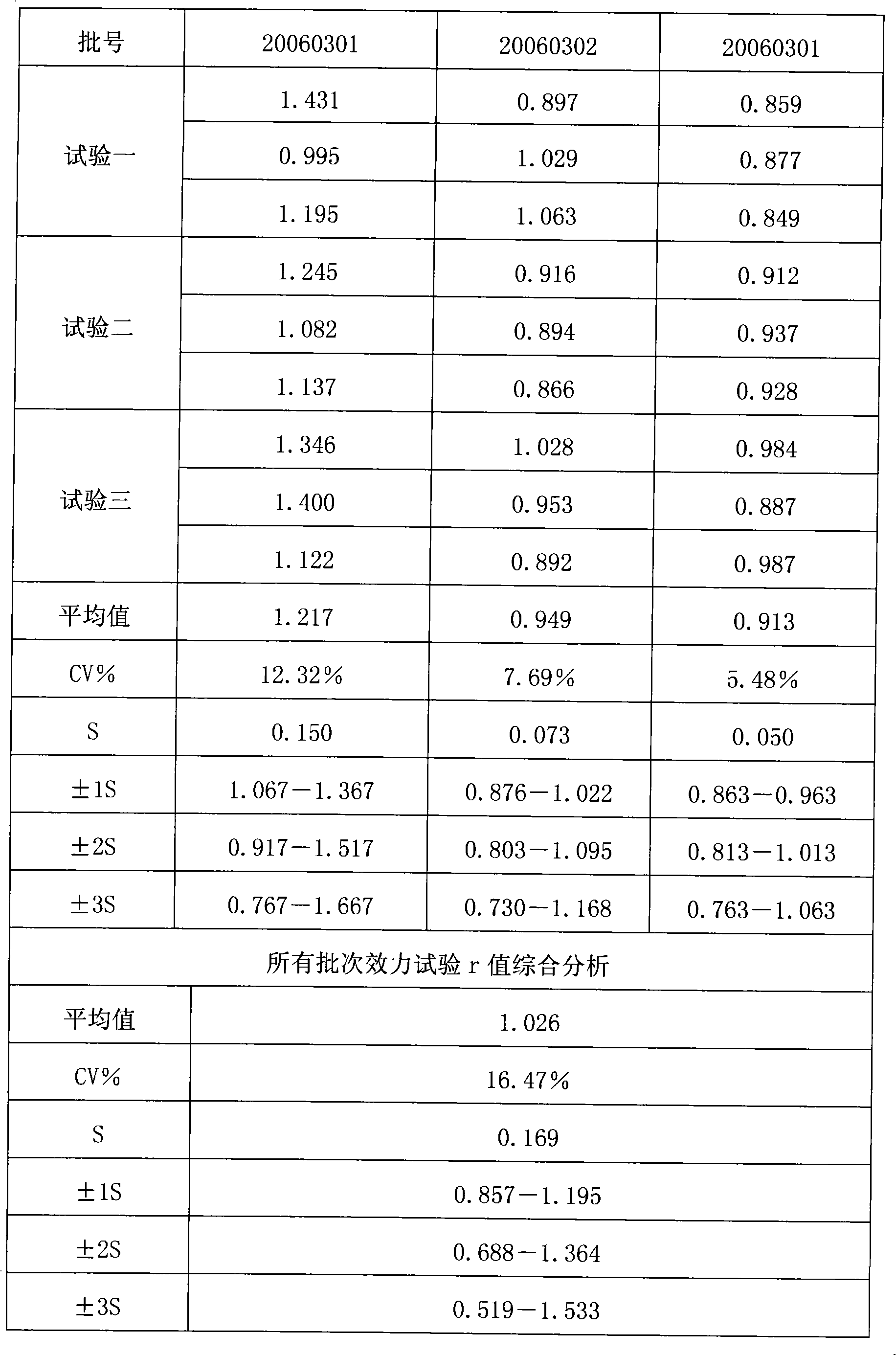
[0036] The reference substance S used in the efficacy test of hepatitis A inactivated vaccine is a qualified product produced by the applicant in strict accordance with national standards, and has reached the standard in chemical and biological tests, and its potency Ps is 640EU / ml; the hepatitis A inactivated vaccine to be tested Live vaccine sample T is also a product produced by the applicant in strict accordance with national standards, and its marked potency is A T It is 640EU / ml.
[0037] The solutions used are as follows:
[0038] 1. Phosphate buffer saline PBS: disodium hydrogen phosphate 58g, potassium dihydrogen phosphate 4g, sodium chloride 160g, add water for injection to 1000ml.
[0039] 2. PBS diluent: Dilute phosphate buffered saline PBS 20 times with water for injection.
[0040] 3. PBS washing solution: After diluting the phosphate buffer saline solution PBS 20 times with water for injection, add 0.05% Tween 20.
[0041] 4. Carbonate buffer solution: 1.6g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com