An improved mercaptolation method for the synthesis of sodium 2,3-dimercaptopropanesulfonate
A technology of sodium dimercaptopropanesulfonate and sulfhydrylation, which is applied in the directions of organic chemistry, thiol preparation, etc., can solve the problems of low sulfhydrylation reaction yield, etc., and achieve the effect of high selectivity and high reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
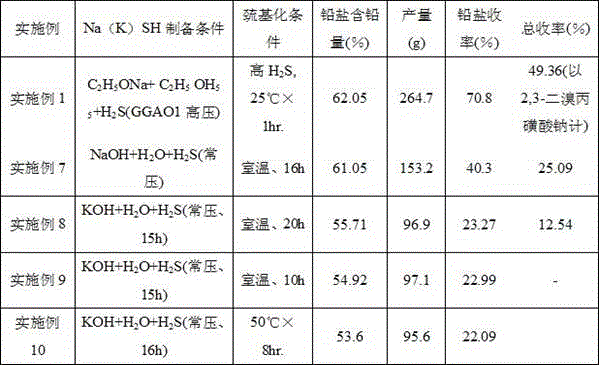
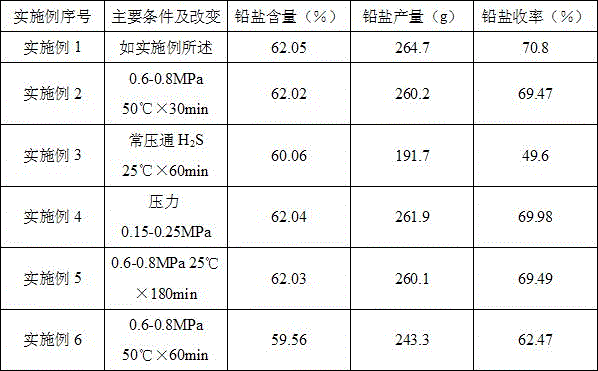
[0034] Embodiment 1 (embodiment of the present invention)
[0035] 1. Preparation of sodium hydrosulfide: 3250ml of absolute ethanol was put into a 5L autoclave, and 110g (1.60mol) of sodium ethoxide was added under stirring. After adding, close and tighten the feeding port, control the internal temperature below 25°C through the jacket cooling water, and feed high-pressure hydrogen sulfide gas into the kettle through the hydrogen sulfide gas cylinder under stirring conditions, and control the pressure within the range of 0.3-0.5MPa Stir the reaction for about 30 minutes, so that the pH value of the solution reaches the range of 6.5-7.5.
[0036] 2. Mercaptolation reaction: After the above reaction is completed, the pressure in the kettle is released, the lid of the kettle is opened, and 230.3g (0.75mol) of sodium 2,3-dibromopropanesulfonate is added at a time. After the addition is complete, tighten the lid of the kettle, and control the internal temperature at 23°C to 25°C...
Embodiment 2(50
[0047] Example 2 (Example of mercaptolation reaction at 50°C)
[0048] In this example, except that the thiolation reaction temperature was changed from room temperature for 90 minutes to 50° C. for 30 minutes, other steps and conditions were exactly the same as those in Example 1 to obtain 260.2 g of lead salt.
[0049] Lead salt content (measured value): 62.02%;
[0050] Lead salt yield: 69.47%.
Embodiment 3
[0051] Embodiment 3 (the pressure control embodiment A of embodiment 1)
[0052] In this example, the other steps and conditions are exactly the same as in Example 1, except that the hydrogen sulfide gas pressure is changed from pressurized to normal pressure during the preparation process of sodium hydrosulfide and the mercaptolation reaction. 191.74 g of lead salt complex was obtained.
[0053] Lead content (measured value): 60.06%;
[0054] Lead salt yield: 49.6%.
[0055] Remarks: when changing the pressurized hydrogen sulfide feeding condition of embodiment 1 into normal pressure feeding, the lead salt quality and yield will obviously decrease.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com