Application of panaxadiol saponin component in preparing antischizophrenic drug
A technology of ginseng diol saponins and schizophrenia, applied in the field of medicine, can solve difficult problems such as medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of ginseng diol saponin components from ginseng rhizome medicinal materials
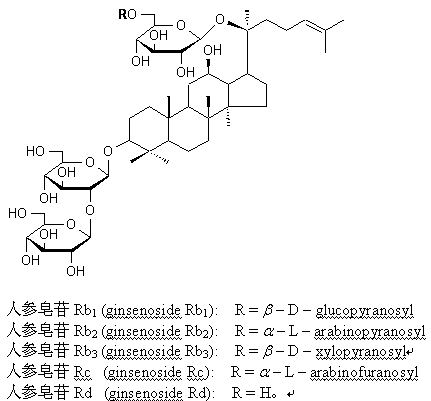
[0020] The ginseng diol saponin component of the present invention is prepared by the separation and purification method in Example 1 in Application No. 201210242928.1. Ginseng or American ginseng rhizomes or leaves are crushed into coarse powder, then extracted by percolation with 50% ethanol aqueous solution until the extract is colorless, combined percolation solution is decompressed to recover ethanol, and freeze-dried to obtain extract. The extract was dissolved in 45% ethanol aqueous solution, and the sample liquid was separated by macroporous adsorption resin column chromatography, first eluted with 45% ethanol aqueous solution until the eluent had no obvious saponin reaction, and then eluted with 70% ethanol aqueous solution to The eluate showed no obvious saponin reaction. The 70% ethanol eluate was decompressed to recover ethanol and freeze-dried to obtain to...
Embodiment 2
[0021] Example 2 Effect of Panaxadiol Saponin Components and Panaxatriol Saponin on the Neuromotor Toxicity of Haloperidol
[0022] 1. Panaxadiol saponins against neuromotor side effects caused by haloperidol
[0023] The reducing effect of panaxadiol saponins (Rb) on the neuromotor toxicity of haloperidol (HAL) was observed by pole climbing test.
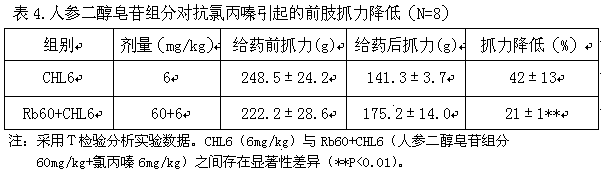
[0024]Male ICR adult mice with a body weight of 30±5g after 6-7 days of acclimatization in the laboratory were randomly and evenly divided into haloperidol (2.0mg / kg, intraperitoneal injection ip) group, panaxadiol saponin (referred to as Rb, 40mg / kg, ip) combined with haloperidol (2.0mg / kg) (referred to as Rb40+HAL) group, panaxadiol saponin (referred to as Rb, 60mg / kg, ip) and haloperidol (2.0mg / kg ) combined (abbreviated as Rb60+HAL) group and ginseng diol saponin (abbreviated as Rb, 80mg / kg, ip) and haloperidol (2.0mg / kg) combined (abbreviated as Rb40+HAL) group, with 8 animals in each group. Rb intraperitoneal administra...
Embodiment 3
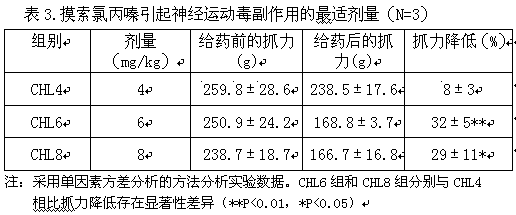
[0034] 1. The dose exploration of ginseng diol saponin components on the neuromotor attenuation effect caused by chlorpromazine
[0035] First explore the appropriate dose of chlorpromazine-induced neuromotor toxicity through the grip test of mice, and then use this dose to determine the influence of ginseng diol saponin components on the neuromotor toxicity caused by chlorpromazine.
[0036] Male ICR adult mice with a body weight of 30±5g after 6-7 days of acclimatization in the laboratory were randomly and evenly divided into three concentration gradient groups of chlorpromazine 4, 6, and 8 mg / kg (intraperitoneal injection, ip), In order to find a suitable dosage, there were 3 mice in each group. The device used to measure myasthenic toxicity in the grip test is the YLS-13A rat grip tester. Measuring conditions: Each mouse was measured 3 times in parallel with an interval of 5 minutes; data processing: reduction in gripping force (%) = [grasping strength before administra...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap