A kind of T-lymphocyte modified by bispecific chimeric antigen receptor and its preparation method and application
A technology of chimeric antigen receptors and lymphocytes, applied in the biological field, to achieve the effect of overcoming off-target toxicity, specific recognition and killing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
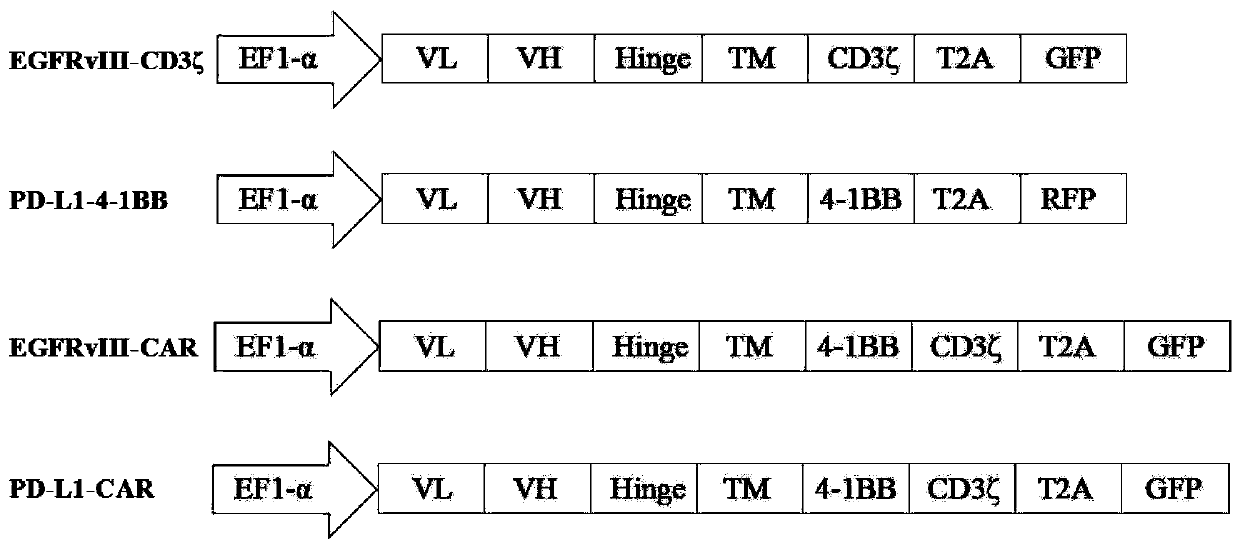
[0058] Example 1 Determination of EGFRvIII-CD3ζ / PD-L1-4-1BB gene sequence
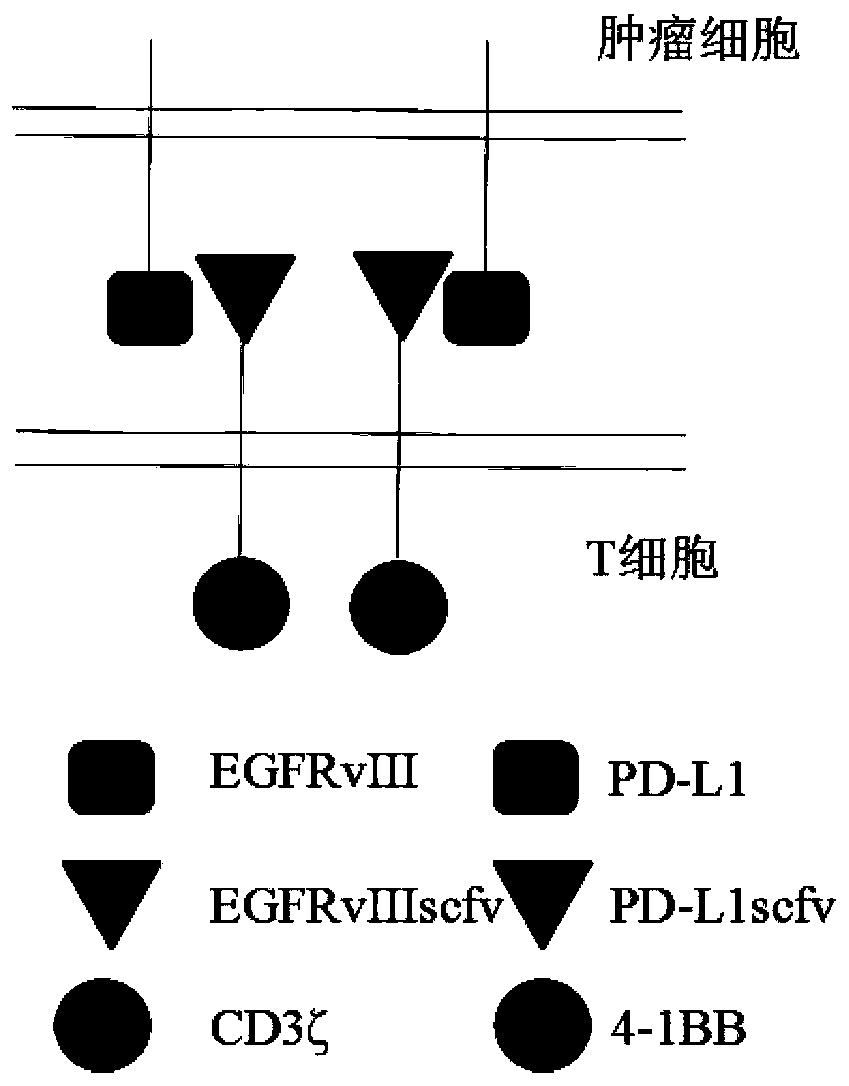
[0059] 1.1 Obtain human CD8α signal peptide gene (SEQ ID NO.9), Linker region (Gly4-Ser) 3 ( SEQ ID NO.10), human CD8α hinge region (CD8α Hinge) (SEQ ID NO.11), human CD8α transmembrane region (CD8α TM) (SEQ ID NO.12), immunoreceptor tyrosine activation motif CD3ζ (SEQ ID NO.11) ID NO.13), human 4-1BB hinge region (4-1BB Hinge) (SEQ ID NO.14), human 4-1BB transmembrane region (4-1BB TM) (SEQ ID NO.1) and 4-1BB Intracellular signal region (SEQ ID NO.16) sequence, combined with EGFRvIII antibody light chain variable region (EGFRvIIIscfv-VL) (SEQ ID NO.17), EGFRvIII antibody heavy chain variable region (EGFRvIIIscfv-VH) (SEQ ID NO. 18), PD-L1 antibody light chain variable region (PD-L1scfv-VL) (SEQ ID NO.19), PD-L1 antibody heavy chain variable region (PD-L1scfv-VH) (SEQ ID NO.20) Sequence, combined to form complete EGFRvIII-CD3ζ (SEQ ID NO.5), PD-L1-4-1BB (SEQ ID NO.6), EGFRvIII-CAR (SEQ ID NO.7), PD-L...
Embodiment 2
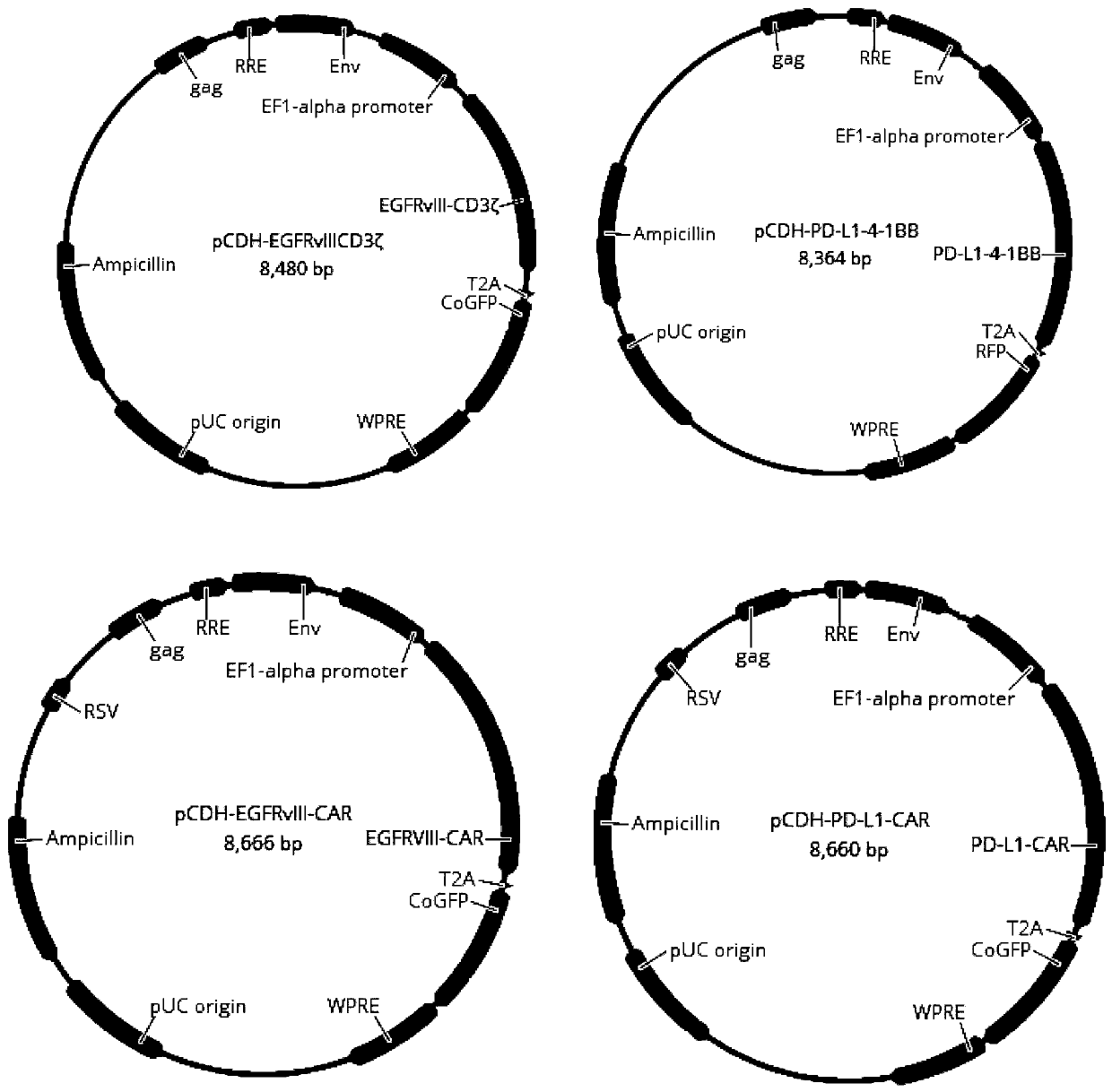
[0060] Example 2 Construction of pCDH-EGFRvIII-CD3ζ, pCDH-PD-L1-4-1BB, pCDH-EGFRvIII-CAR and pCDH-PD-L1-CAR plasmids
[0061] 2.1 Whole gene synthesis:
[0062]Complete EGFRvIII-CD3ζ (SEQ ID NO.5), PD-L1-4-1BB (SEQ ID NO.6), EGFRvIII-CAR (SEQ ID NO.7), PD- L1-CAR (SEQ ID NO.8) sequence, and add Xba I restriction site at its 5' end, and add EcoR I restriction site at its 3' end.
[0063] 2.2 Cloning EGFRvIII-CD3ζ, PD-L1-4-1BB, EGFRvIII-CAR, and PD-L1-CAR into pCDH-EF1α-GFP or pCDH-EF1α-RFP lentiviral expression vectors, respectively. The details are as follows: the pCDH-EF1α-GFP vector, pCDH-EF1α-RFP vector, EGFRvIII-CD3ζ fragment, PD-L1-4-1BB fragment, EGFRvIII-CAR fragment, and PD-L1-CAR fragment were respectively treated with Xba I / EcoR I Digest with enzymes, and recover 8192bp, 8111bp, 1297bp, 1258bp, 1483bp, 1477bp fragments respectively. Use T4 DNA ligase to ligate pCDH-EF1α-GFP vector and EGFRvIII-CD3ζ, EGFRvIII-CAR, PD-L1-CAR, pCDH-EF1α-RFP vector and PD-L1-4-1BB res...
Embodiment 3
[0064] Packaging, concentration and titer determination of embodiment 3 lentivirus
[0065] 3.1 Packaging of lentivirus:
[0066] 3.1.1 Cell treatment: 24 hours before transfection, collect 293T cells of passage 3-10 in logarithmic growth phase, inoculate 293T cells in a 10cm cell culture dish, the inoculum size is 1×10^7, and the cells are contained in 10ml 10 Grow in DMEM medium with %FBS, place in a 5% CO2 cell incubator at 37°C for 18 hours, and transfect when the cell density reaches 60-80%.
[0067] 3.1.2 Co-transfect lentiviral expression vector plasmids (pCDH-EGFRvIII-CD3ζ, pCDH-PD-L1-4-1BB, pCDH-EGFRvIII-CAR, pCDH-PD-L1-CAR) and their packaging plasmids ( pLP1, pLP2, pLP / VSVG);
[0068] 3.1.3 24 hours after transfection, the expression of GFP / RFP fluorescence in 293T cells after transfection was observed under a fluorescent microscope. Collect the 293T culture supernatant at 48 hours and 72 hours after transfection, centrifuge at 3000rpm for 15 minutes, collect the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com