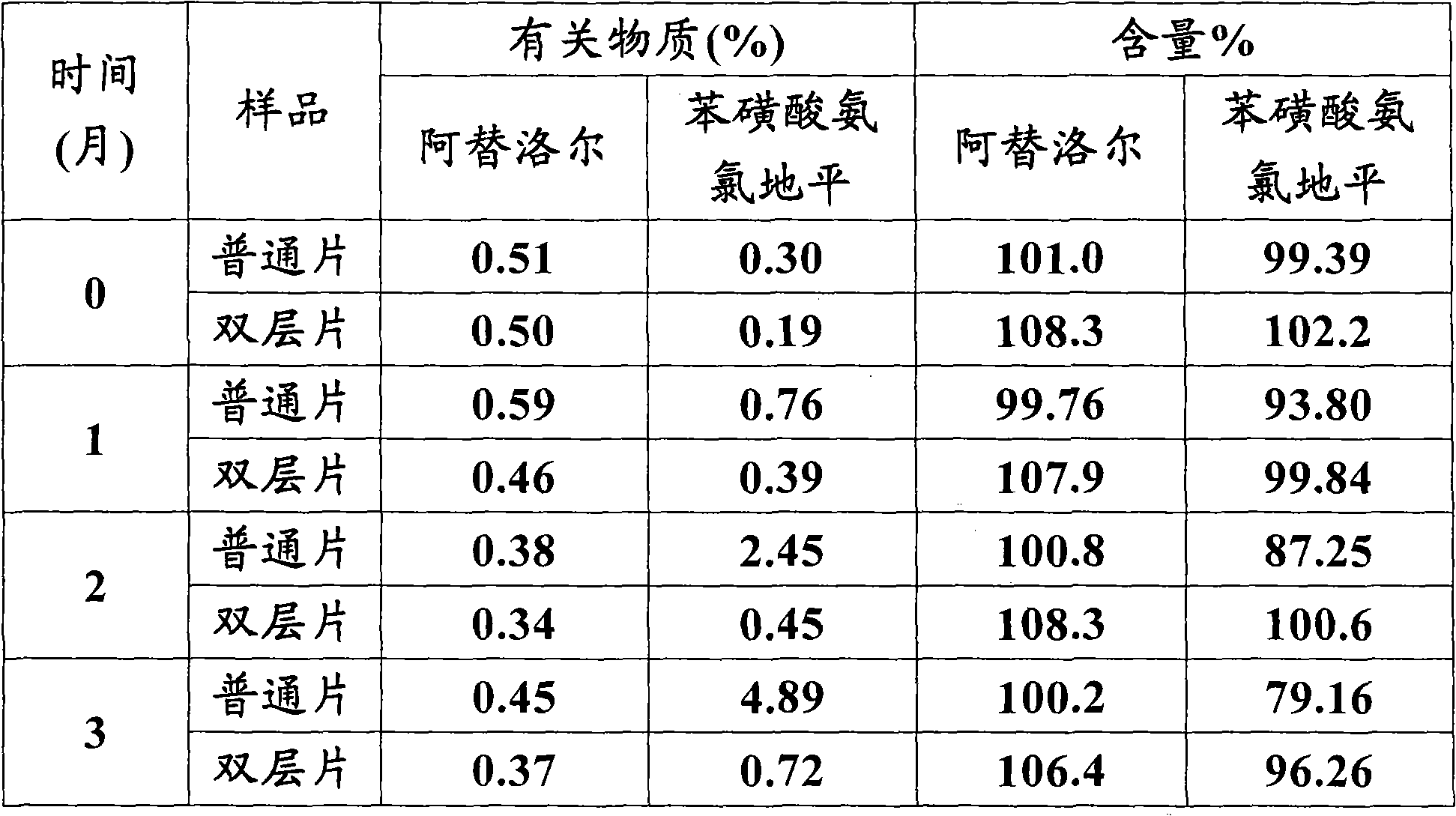
Atenolol and amlodipine bilayer tablet
A double-layer tablet, amlodipine technology, applied in the field of medicine, can solve the problems of unqualified dissolution rate and uneven dissolution rate of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of atenolol-amlodipine bilayer tablet
[0056] Plain Tablet Prescription:
[0057] (1) Atenolol layer:
[0058] atenolol
10mg,
Calcium hydrogen phosphate
50mg,
microcrystalline cellulose
7.5 mg,
Sodium Carboxymethyl Starch
2.5 mg,
pvp K30
Appropriate amount,
Magnesium stearate
0.35 mg;
[0059] (2) Amlodipine layer:
[0060] Amlodipine
1mg (in bases),
Calcium hydrogen phosphate
50mg,
microcrystalline cellulose
7.5 mg,
Sodium Carboxymethyl Starch
0.75mg,
pvp K30
Appropriate amount,
Magnesium stearate
0.35 mg.
[0061] Prescription of film coating solution (according to 1000ml):
[0062] Hydroxypropyl methylcellulose 20.0 g, Tween-808.0 g, talc 15.0 g, titanium dioxide 10.0 g, and the solvent is 50% ethanol.
[0063] Preparation Process:
[0064] Take the prescription amount of at...
Embodiment 2
[0065] Embodiment 2: Preparation of atenolol-amlodipine bilayer tablet
[0066] Plain Tablet Prescription:
[0067] (1) Atenolol layer:
[0068] atenolol
15mg,
Calcium hydrogen phosphate
40mg,
microcrystalline cellulose
10mg,
Sodium Carboxymethyl Starch
2mg,
pvp K30
Appropriate amount,
Magnesium stearate
0.3mg;
[0069] (2) Amlodipine layer:
[0070] Amlodipine
2mg (based on base),
Calcium hydrogen phosphate
40mg,
microcrystalline cellulose
10 mg,
[0071] Sodium Carboxymethyl Starch
0.5mg,
pvp K30
Appropriate amount,
Magnesium stearate
0.3 mg.
[0072] Preparation Process:
[0073] According to the above-listed formulation, tableting was carried out basically according to the preparation process of Example 1, and the prepared bilayer tablet was coated.
Embodiment 3
[0074] Embodiment 3: Preparation of atenolol-amlodipine bilayer tablet
[0075] Plain Tablet Prescription:
[0076] (1) Atenolol layer:
[0077] atenolol
5mg,
Calcium hydrogen phosphate
60mg,
microcrystalline cellulose
5mg,
Sodium Carboxymethyl Starch
3mg,
PVP K30
Appropriate amount,
Magnesium stearate
0.4mg;
[0078] (2) Amlodipine layer:
[0079] Amlodipine
1mg (in bases),
Calcium hydrogen phosphate
60mg,
microcrystalline cellulose
5 mg,
Sodium Carboxymethyl Starch
1.0mg,
PVP K30
Appropriate amount,
Magnesium stearate
0.4mg.
[0080] Preparation Process:
[0081] According to the above-listed prescription, the tableting is basically carried out according to the preparation process of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com