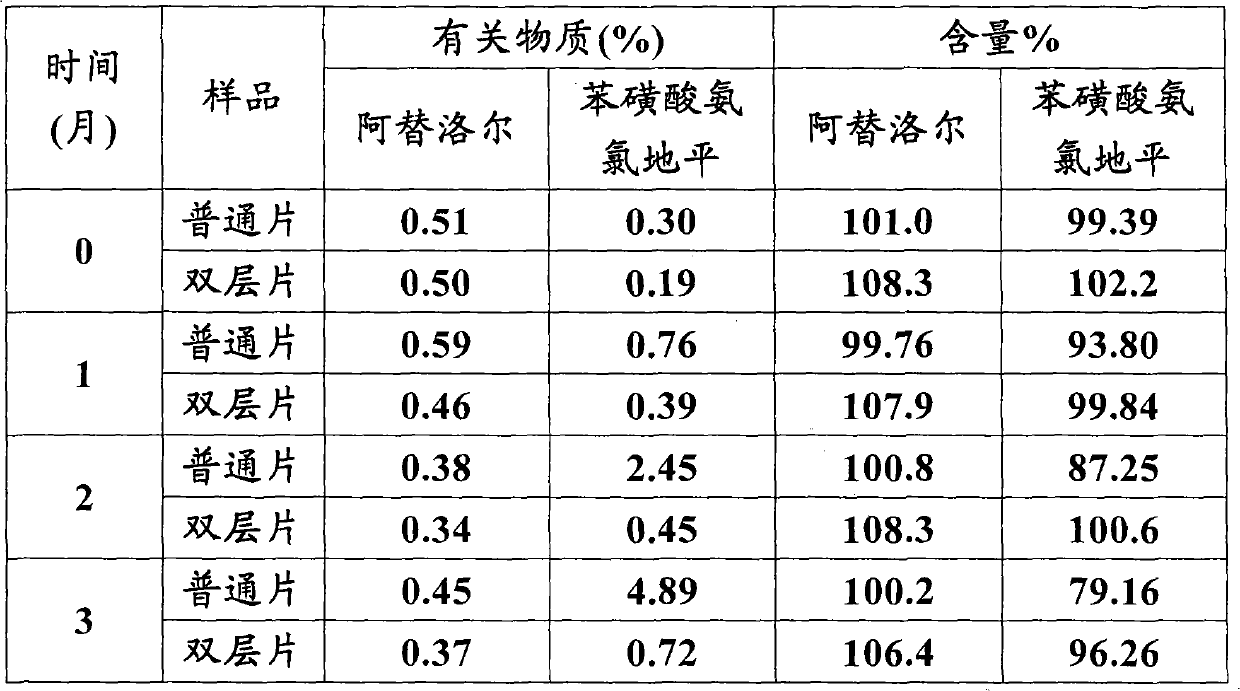
Atenolol and amlodipine bilayer tablet
A double-layer tablet, amlodipine technology, applied in the field of medicine, can solve the problems of unqualified dissolution and uneven dissolution of active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: Preparation of atenolol-amlodipine bilayer tablet
[0055] Plain Tablet Prescription:
[0056] (1) Atenolol layer:
[0057] Atenolol
10 mg,
50mg,
7.5 mg,
2.5 mg,
pvp K30
Appropriate amount,
0.35 mg;
[0058] (2) Amlodipine layer:
[0059] Amlodipine
1mg (base-based),
50mg,
7.5 mg,
0.75mg,
pvp K30
Appropriate amount,
0.35 mg.
[0060] Prescription of film coating solution (according to 1000ml):
[0061] Hydroxypropyl methylcellulose 20.0g, Tween-808.0g, talcum powder 15.0g, titanium dioxide 10.0g, solvent is 50% ethanol.
[0062] Preparation Process:
[0063] Take prescription amount of atenolol,...
Embodiment 2
[0064] Embodiment 2: the preparation of atenolol-amlodipine bilayer tablet
[0065] Plain Tablet Prescription:
[0066] (1) Atenolol layer:
[0067] Atenolol
15 mg,
40mg,
10 mg,
2 mg,
pvp K30
Appropriate amount,
0.3 mg;
[0068] (2) Amlodipine layer:
[0069] Amlodipine
2mg (calculated by base),
Calcium hydrogen phosphate
40mg,
10mg,
0.5 mg,
pvp K30
Appropriate amount,
0.3 mg.
[0070] Preparation Process:
[0071] According to the prescription listed above, basically carry out tablet making according to the preparation process of Example 1, and the double-layer tablet coating prepared.
Embodiment 3
[0072] Embodiment 3: Preparation of atenolol-amlodipine bilayer tablet
[0073] Plain Tablet Prescription:
[0074] (1) Atenolol layer:
[0075] Atenolol
5 mg,
Calcium hydrogen phosphate
60mg,
5 mg,
3 mg,
pvp K30
Appropriate amount,
0.4 mg;
[0076] (2) Amlodipine layer:
[0077] Amlodipine
1mg (base-based),
Calcium hydrogen phosphate
60mg,
5 mg,
1.0 mg,
pvp K30
Appropriate amount,
0.4mg.
[0078] Preparation Process:
[0079] According to the prescription listed above, basically carry out tablet making according to the preparation technology of embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com