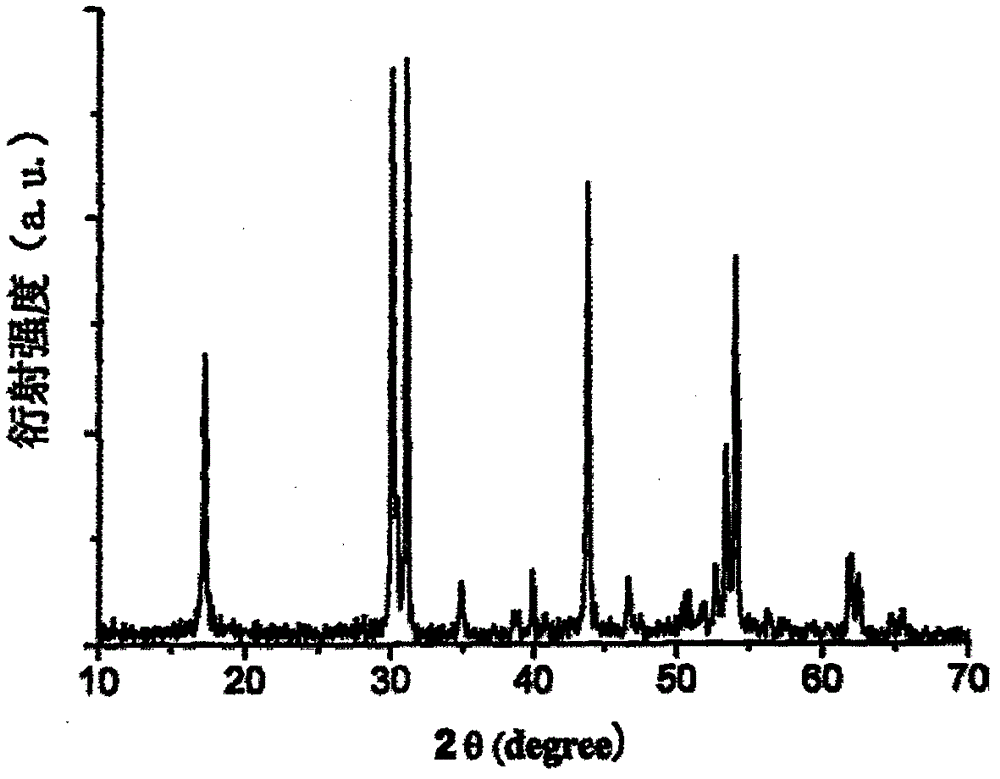
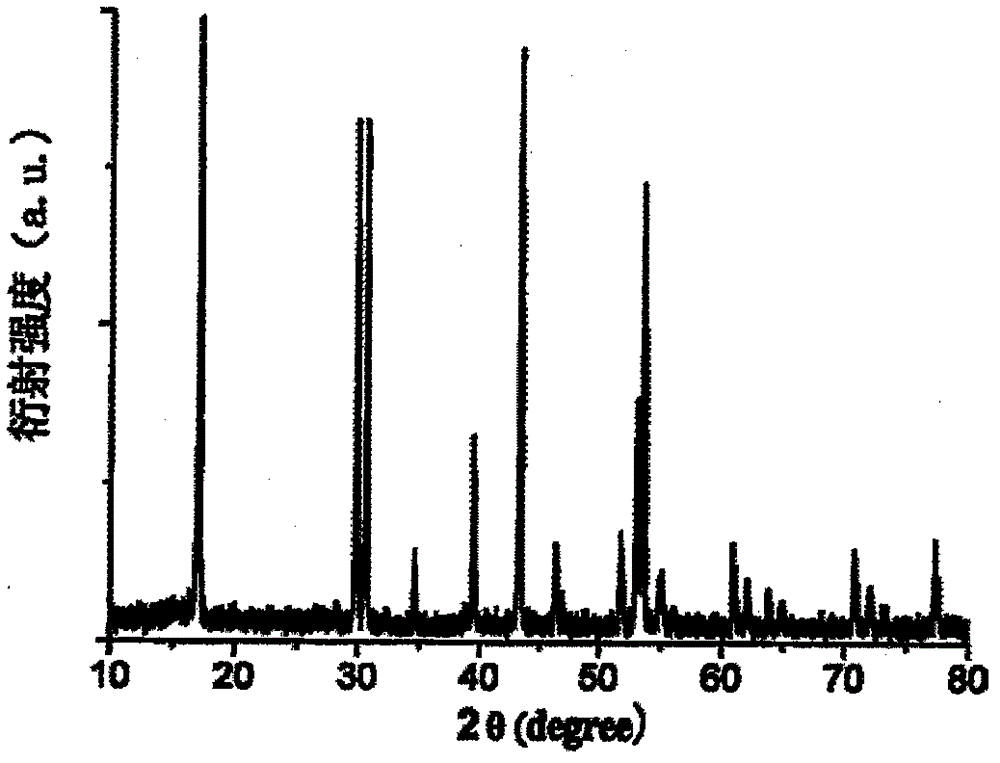
Preparation method of rare earth fluoride in air
A rare earth fluoride and fluoride technology, applied in the field of rare earth fluoride, can solve the problems of danger, expensive raw materials, high pressure, etc., achieve good water and acid resistance and stability, simple preparation method, and reduce the effect of sintering temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The molecular formula of synthetic fluoride is NaYbF 4 . Weigh 2.1731 g Yb 2 o 3 , dissolved with nitric acid to form a rare earth salt solution, and ammonium bicarbonate was added to adjust the pH value of the rare earth salt solution to 6. Then weigh 1.8873 grams of precipitant NH 4 F, and dissolved in deionized water to prepare a precipitant solution. Add excess precipitant solution to rare earth salt solution at room temperature, and magnetically stir the mixed solution. Stop stirring, stand still, get rare earth fluoride YbF 3 precipitation. Wash the precipitate by adding deionized water several times by decantation until the pH value of the washing solution is 7. Precipitate YbF 3 Add to distilled water and stir magnetically to form a suspension. Add 0.4631 g of NaF solution to the suspension to obtain a mixed solution. In an atmosphere without hydrogen fluoride in the air, and magnetically stirred, the mixture was heated at 80°C for 36 hours to obtain n...
Embodiment 2
[0034] The molecular formula of synthetic fluoride is LiYbF 4 . Weigh 2.3093 g Yb 2 o 3 , dissolved with hydrochloric acid to form a rare earth salt solution, adding ammonia water to adjust the pH value of the rare earth salt solution to 6.5. Then weigh 1.2 g of precipitant NH 4 HF 2 , and dissolved in deionized water to prepare a precipitant solution. Add excess precipitant solution to rare earth salt solution at room temperature, and magnetically stir the mixed solution. Stop stirring, stand still, get rare earth fluoride YbF 3 precipitation. Use a high-speed centrifuge to separate and wash the precipitate until the pH of the washing solution is 7. Precipitate YbF 3 Add to distilled water and stir magnetically to form a suspension. Add 0.3040 g of liF solution to the suspension to obtain a mixed solution. In the air without hydrogen fluoride atmosphere, and magnetic stirring, the mixture was heated at 120°C for 72 hours to obtain nano-sized LiYbF with a particle s...
Embodiment 3
[0036] The molecular formula of synthetic fluoride is NaYb 0.92 Er 0.08 f 4 . Weigh 2.0026 g Yb 2 o 3 and 0.1691 g Er 2 o 3 , Dissolve with hydrochloric acid, and heat the solution to evaporate water to make it close to dryness, then add deionized water to dilute to form a rare earth salt solution. Then weigh 1.8905 g of precipitant NH 4 HF 2 , and dissolved in deionized water to prepare a precipitant solution. Add excess precipitant solution to rare earth salt solution at room temperature, and magnetically stir the mixed solution. Stop stirring, stand still, get rare earth fluoride Yb 0.92 Er 0.08 f 3 precipitation. Use a high-speed centrifuge to separate and wash the precipitate until the pH of the washing solution is 7. Precipitate Yb 0.92 Er 0.08 f 3 Add to distilled water and stir magnetically to form a suspension. Add 0.4639 g of NaF solution to the suspension to obtain a mixed solution. In an atmosphere without hydrogen fluoride in the air, and magnet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com