Synthetic method of spirostaline alkane glycoalkaloids
A kind of technology of spiro alkane and synthesis method, which is applied in the field of drug synthesis and can solve the problems of low efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
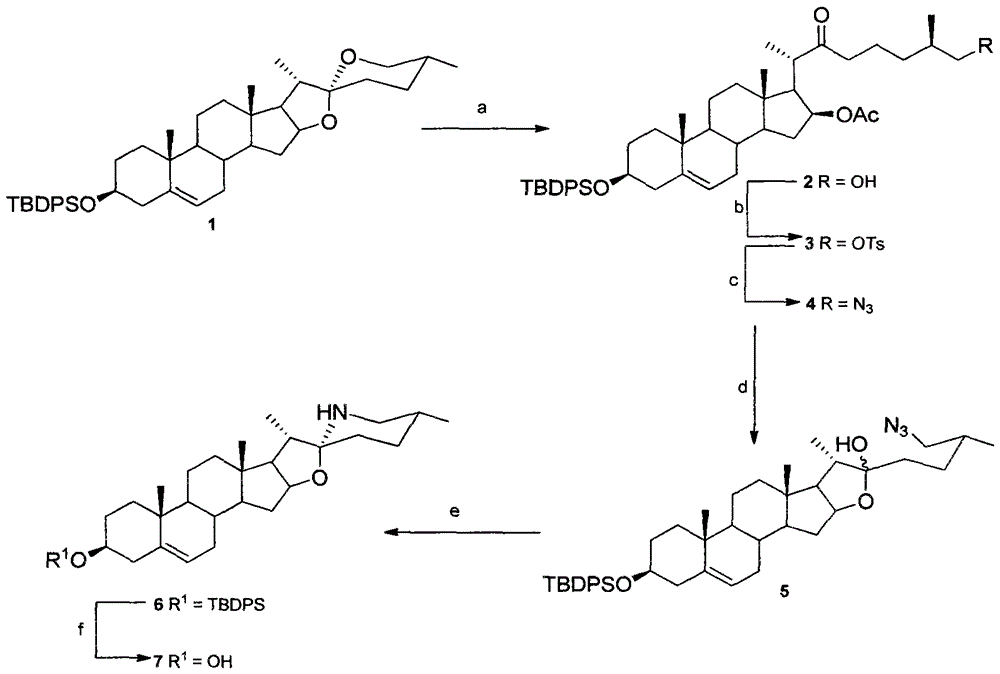
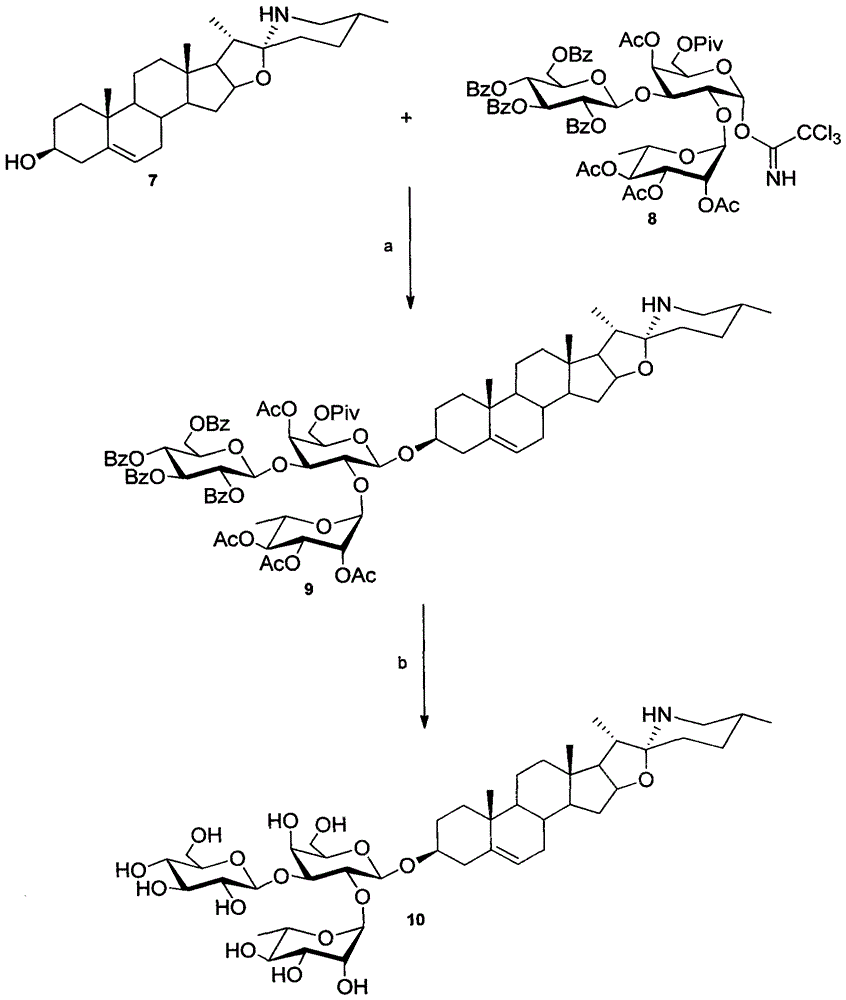
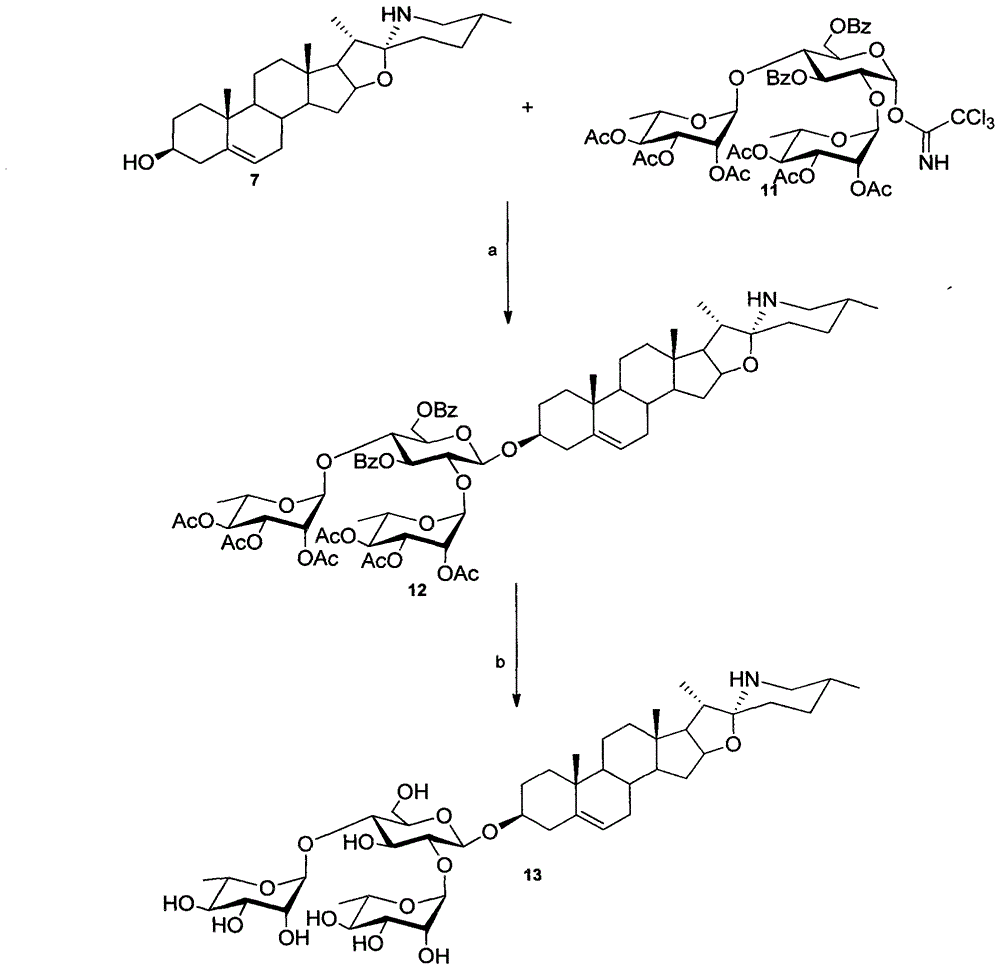
[0077] The present invention will be described in detail below with reference to the accompanying drawings and specific examples of solamargine and solasodine, but the present invention is not limited to the following content.
[0078] Preparation and data of compound 2
[0079] Diosgenin 1 (3.0g, 4.60mmol), dichloromethane (30mL), glacial acetic acid (3.3mL, 32.2mmol) were added successively in a single-necked bottle, and under argon protection, the After stirring in ice bath for 20min, slowly add BF dropwise 3 ·OEt 2 , the dropwise addition was completed within 25 minutes, the reaction product was immediately transferred to ice water, stirred to make it fully mixed; then extracted with dichloromethane, collected and combined the organic phase, then washed with saturated sodium bicarbonate and saturated brine, and washed with anhydrous sulfuric acid Sodium was dried and filtered, and the filtrate was concentrated and then silica gel column chromatography (CH 2 Cl 2 : ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com