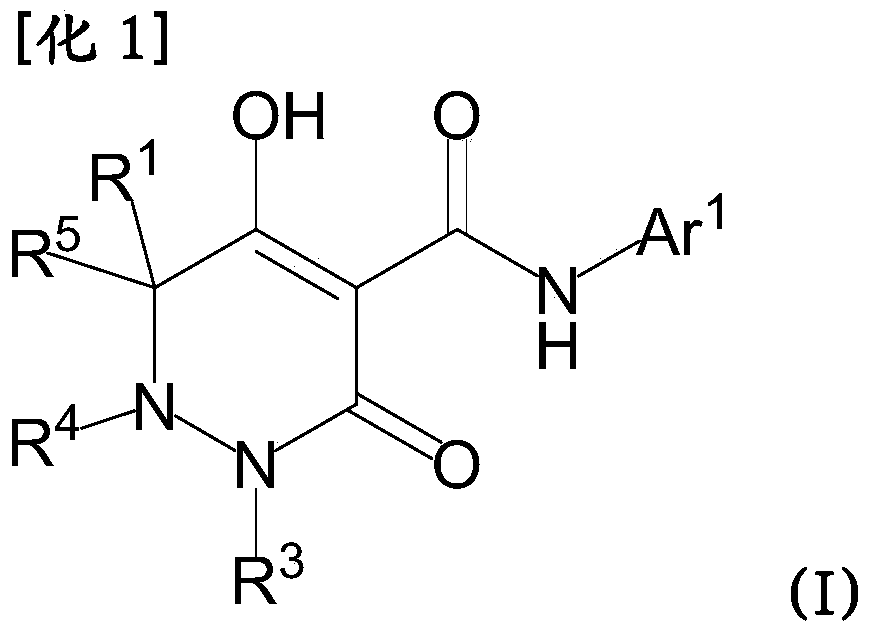
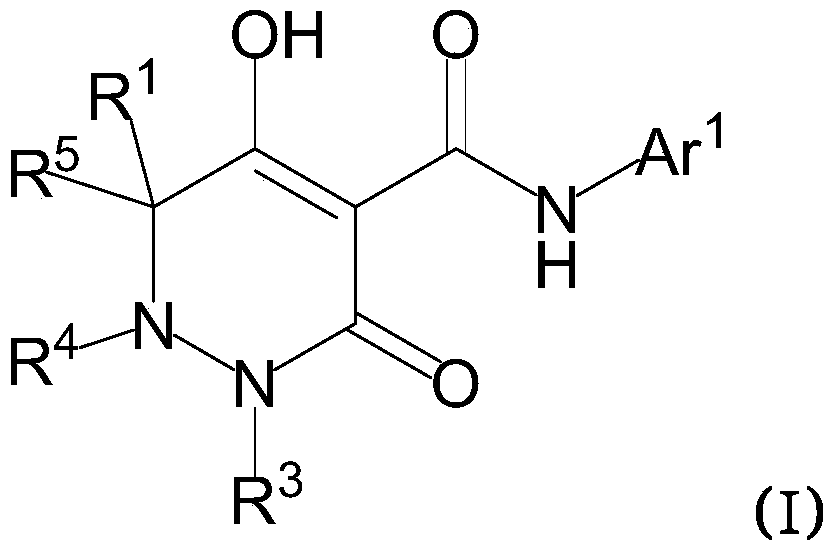
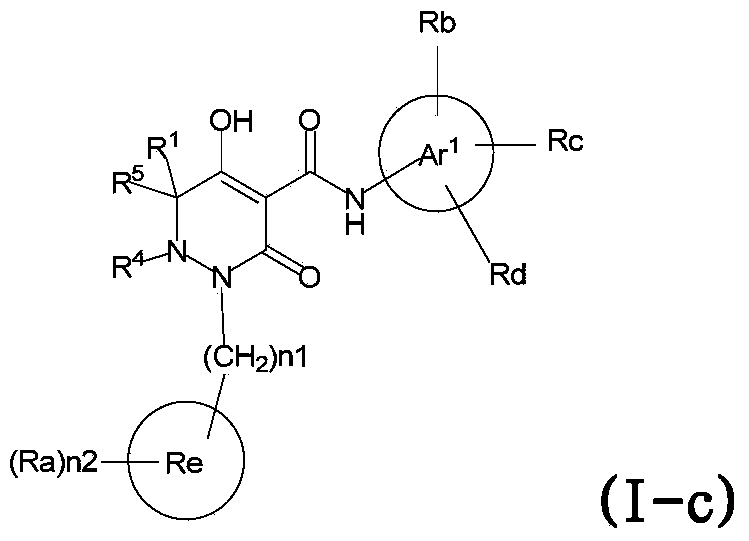
Dihydropyridazine-3,5-dione derivative
A compound and hydrogen atom technology, applied in drug combination, active ingredient of heterocyclic compound, metabolic disease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0973] (4aS)-1-[(3-fluorophenyl)methyl]-4-hydroxyl-N-[5-methyl-2-(trifluoromethyl)furan-3-yl]-2-oxo -4a,5,6,7-tetrahydropyrrolo[1,2-b]pyridazine-3-carboxamide
[0974] first step
[0975] (S)-1-[(3-Fluoro-benzylidene)-amino]-pyrrolidine-2-carboxylic acid methyl ester
[0976] [chem 29]
[0977]
[0978] L-proline
[0979] Methyl ester hydrochloride (5.00g, 30.2mmol) was suspended in dichloromethane (60.4mL), p-toluenesulfonic acid, monohydrate (6.03g, 31.7mmol) was added, under nitrogen, at room temperature for 10 Stir for minutes. The reaction mixture was concentrated under reduced pressure, added toluene to make it azeotrope, suspended in dichloromethane (60.4 mL), added sodium nitrite (2.19 g, 31.7 mmol), and stirred at room temperature for 2 hours under a nitrogen atmosphere. After filtering the reaction mixture, it was concentrated under reduced pressure to obtain a crude product of (S)-1-nitroso-pyrrolidine-2-carboxylic acid methyl ester. The obtained crude prod...
Embodiment 2
[0997] (4aR)-1-[(3-fluorophenyl)methyl]-4-hydroxyl-N-[5-methyl-2-(trifluoromethyl)furan-3-yl]-2-oxo -4a,5,6,7-tetrahydropyrrolo[1,2-b]pyridazine-3-carboxamide
[0998] In the same manner as in the first to third steps of Example 1, the compounds shown in the table below were synthesized from D-proline methyl ester hydrochloride and 3-fluoro-benzaldehyde.
[0999] [Table 2]
[1000]
[1001]
Embodiment 3
[1003] (4aS)-1-[(3-fluorophenyl)methyl]-4-hydroxy-6,6-dimethyl-N-[5-methyl-2-(trifluoromethyl)furan-3- Base]-2-oxo-5,7-dihydro-4aH-pyrrolo[1,2-b]pyridazine-3-carboxamide
[1004] first step
[1005] (S)-4,4-Dimethyl-pyrrolidine-2-carboxylic acid ethyl ester hydrochloride
[1006] [chem 32]
[1007]
[1008] To (S)-4,4-dimethyl-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester 2-ethyl ester (500mg, 1.84mmol) added hydrogen chloride in 1,4-diox An alkane solution (4M, 5.00 mL) was stirred at room temperature for 1 hour under a nitrogen atmosphere. The reaction mixture was concentrated under reduced pressure, and toluene was added to make it azeotrope to obtain a crude product of the title compound.
[1009] second step
[1010] In the same manner as in the first to third steps of Example 1, the obtained (S)-4,4-dimethyl-pyrrolidine-2-carboxylic acid ethyl ester hydrochloride and 3-fluoro-benzaldehyde Synthesize the following compounds.
[1011] [table 3]
[1012] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com