Use of T0901317 as a PARP1 inhibitor
A use, technology of trifluoroethyl, applied in the field of application of PARP1 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Detection by Cell-Free PARP1 Enzymatic Reaction System
[0027] The PARP1 activity detection kit was used to construct a cell-free PARP1 enzymatic reaction system, which can activate the activity of PARP1 enzyme in vitro.
[0028] The PARP1 activity detection kit used in the examples was purchased from Trevigen Company, produced in the United States, and the model number is 4676-096-K.
[0029] PARP1 activity detection kit includes buffer, PARP1 recombinant protein, NAD + , single-strand break DNA, recombinant histone 1 (Histone H1).
[0030] Configure the cell-free PARP1 enzymatic reaction system according to the PARP1 activity detection kit. The specific operation steps are as follows: add 50ng PARP1 recombinant protein (PARP1protein) to the buffer solution with a final concentration of 10mmol / L NAD + , The final concentration is 20mg / mL after single-strand break DNA. The final concentration of each component of the buffer solution in the reaction system ...
Embodiment 2
[0037] Embodiment 2 utilizes in vitro cell experiment to detect
[0038] In the in vitro cell experiment, HepG2 (derived from American Type Culture Collection) cells were selected as experimental cells. The inventors treated HepG2 cells with different concentrations of T09013171. For treatment methods, see Huang D, Yang C, WangY, Liao Y, & Huang K. PARP-1 suppresses adiponectin expression through poly(ADP-ribosyl)ation of PPAR gamma in cardiac fibroblasts. Cardiovascular research, 2009, 81(1): 98-107 .
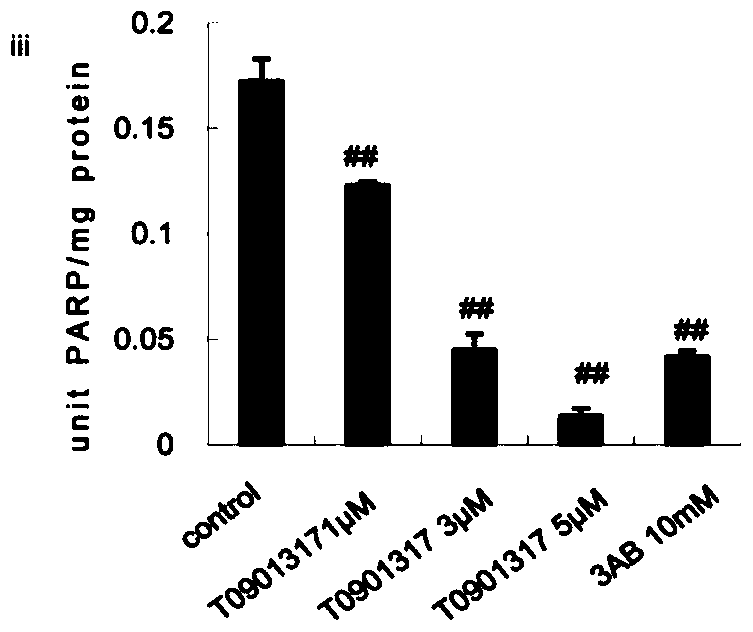
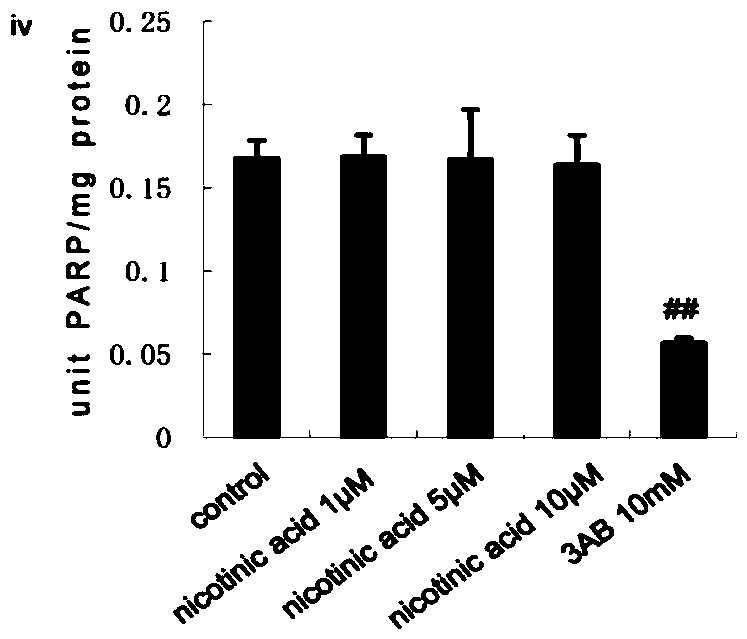
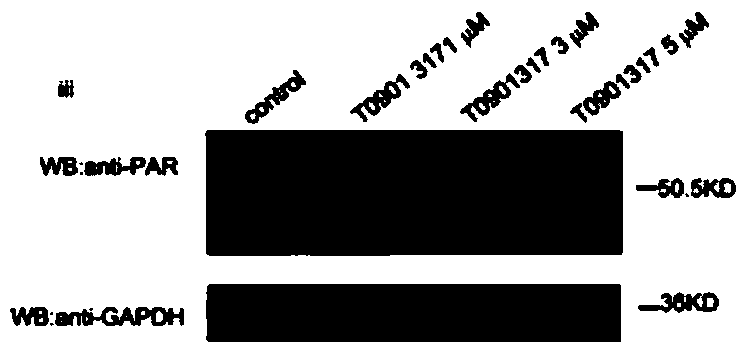
[0039] HepG2 cells were stimulated with T0901317 (iii, 1 μmol / L, 3 μmol / L, 5 μmol / L) or nicotinic acid (iv, 1 μmol / L, 5 μmol / L, 10 μmol / L) for 24 hours, and the concentrations in the above brackets were final concentration.
[0040] PARP1 activity was detected using a PARP1 activity detection kit (purchased from Trevigen, produced in the United States, model number 4676-096-K). For specific methods, refer to the instructions of the kit. Among them, 3AB (10 mmol / L) was used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com