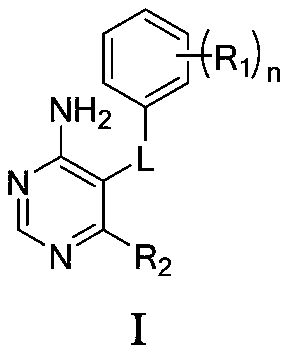
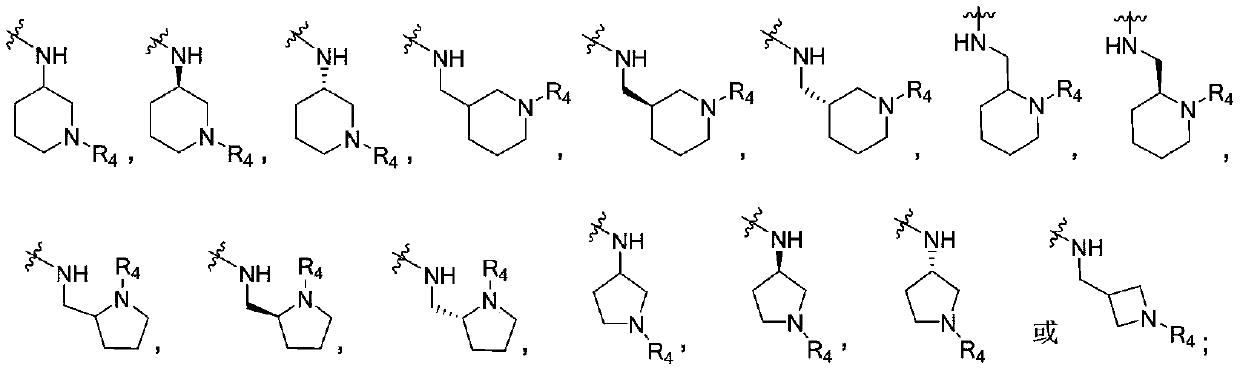
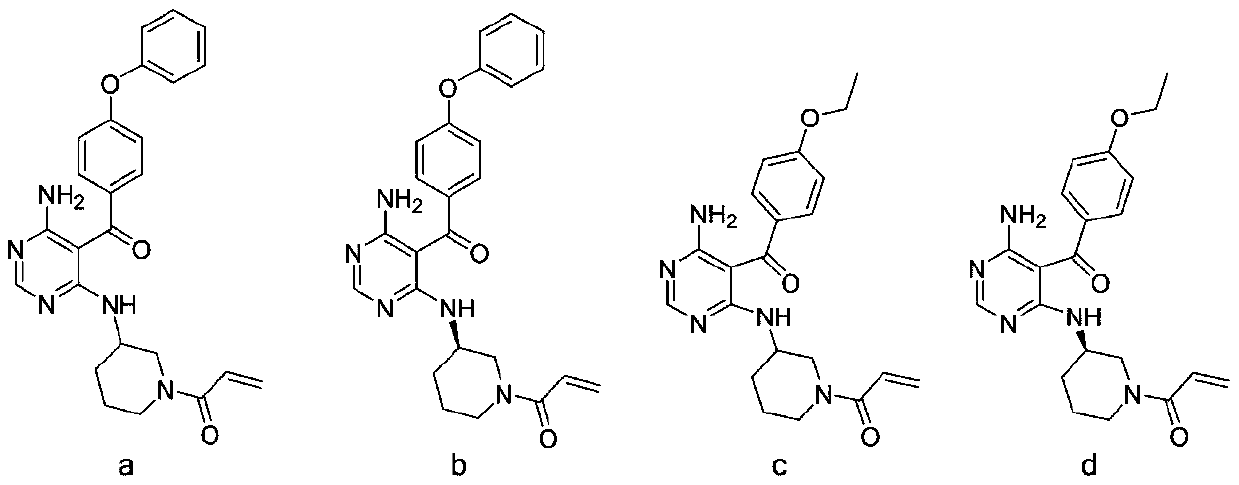
4-aminopyrimidine compound, its preparation method and medical application
A technology of aminopyrimidines and compounds, which can be used in pharmaceutical formulations, antipyretics, drug combinations, etc., and can solve the problem of few types of BTK inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Preparation of 4,6-dichloro-5-pyrimidinecarbaldehyde (1)
[0087] DMF (5.50mL, 71.34mmol) was slowly dropped into POCl under ice bath 3 (17.00mL, 185.71mmol), stirred for 1h, removed the ice bath, added 4,6-dihydroxypyrimidine (4.00g, 35.68mmol), heated and refluxed for 3h, cooled to room temperature, poured into ice water, extracted with dichloromethane, Concentrate under reduced pressure, and recrystallize from petroleum ether-ethyl acetate (P:E=4:1(V:V)) to obtain 4.74 g of yellow solid, yield 75.4%, mp 68-70°C. ESI-MS:177[M+H] + ; 1 H NMR (300MHz, CDCl 3 ): δ (ppm): 8.89 (s, 1H), 10.43 (s, 1H).
[0088] Preparation of 4,6-dichloro-5-pyrimidinecarboxylic acid (2)
[0089] 1 (4.00g, 22.72mmol) and NaH 2 PO 4 (9.55g, 79.58mmol) was dissolved in a mixed solvent of 60mL tert-butanol and 10mL water, and NaClO was added under ice-cooling 2 (7.66g, 84.69mmol), reacted for 1h, distilled off tert-butanol under reduced pressure, poured into water, adjusted pH to 5 with...
Embodiment 2
[0100] Preparation of (R)-3-[[6-amino-5-(4-phenoxybenzoyl)pyrimidin-4-yl]aminomethyl]piperidine-1-carboxylic acid tert-butyl ester (5b)
[0101] Referring to the preparation method of 5a, a pale yellow solid was obtained from the reaction of 4 and (R)-1-Boc-3-aminomethylpiperidine with a yield of 56.2%. ESI-MS:504[M+H] + .
[0102] (R)-1-[3-[[5-(4-phenoxybenzoyl)-6-aminopyrimidin-4-yl]aminomethyl]piperidin-1-yl]-2-propene-1 - Preparation of ketone (A-2)
[0103]
[0104] Referring to the preparation method of A-1, a yellow solid was obtained by reacting 5b with acryloyl chloride after removing the Boc protecting group, with a yield of 17.2%. ESI-MS:458[M+H] + ; 1 H NMR (300MHz, DMSO-d 6 ):δ(ppm):7.97(s,1H),7.65(d,J=8.2Hz,2H),7.39(d,J=8.7Hz,2H),7.19(s,1H),7.09-6.94(m ,4H),6.64(s,3H),5.99(d,J=16.7Hz,1H),5.57(s,1H),4.20-3.62(m,3H),3.18(s,2H),2.98(s, 1H), 2.74(s, 1H), 1.57(s, 3H), 1.16(d, J=24.2Hz, 2H).
Embodiment 3
[0106] Preparation of [4-amino-6-[(piperidine-2-methyl)amino]pyrimidin-5-yl](4-phenoxyphenyl)methanone (5c)
[0107] Referring to the preparation method of 5a, a pale yellow solid was obtained from the reaction of 4 and 2-aminomethylpiperidine with a yield of 45.8%. ESI-MS:404[M+H] + .
[0108] 1-[2-[[5-(4-phenoxybenzoyl)-6-aminopyrimidin-4-yl]aminomethyl]piperidin-1-yl]-2-propen-1-one (A -3) Preparation
[0109]
[0110] 5c (0.23g, 0.57mmol) was dissolved in 18mL tetrahydrofuran-water (THF:H 2 O=5:1(V:V)) mixed system, add NaHCO 3 (0.14g, 1.71mmol), acryloyl chloride (0.06mL, 0.68mmol) was added dropwise under ice bath conditions, reacted at room temperature overnight, THF was distilled off under reduced pressure, an appropriate amount of water was added to the remaining reaction solution, CH 2 Cl 2 Extracted, dried over anhydrous magnesium sulfate, and purified by column chromatography [ethyl acetate (EA)] to obtain 0.04 g of a yellow solid with a yield of 16.0%. E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com