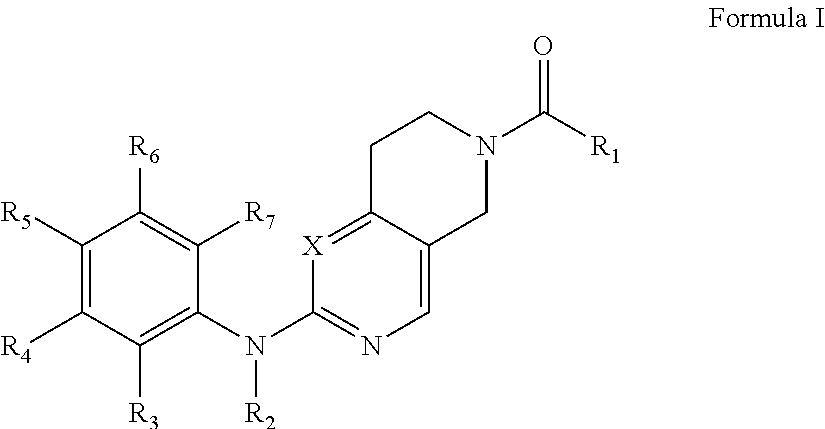
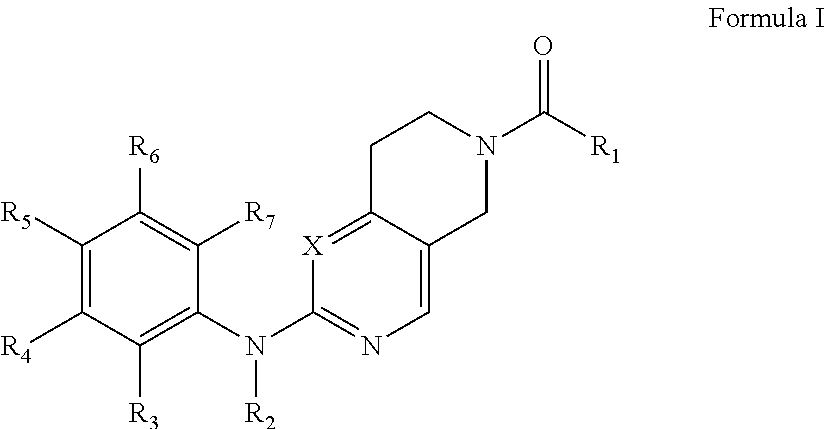
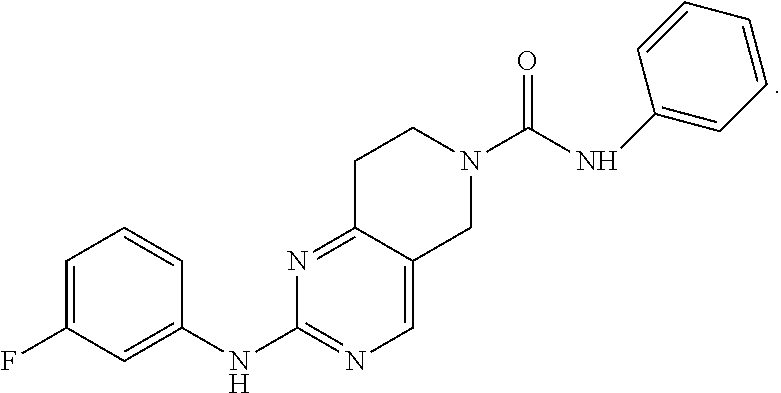
Piperidylpyrimidine derivatives as modulators of protein kinase inhibitors and of vascular endothelial growth factor receptor 2
a technology of vascular endothelial growth factor and piperidylpyrimidine, which is applied in the direction of biocide, organic chemistry, animal husbandry, etc., can solve the problems of abnormal blood vessel growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0221]
6-(Pyridin-3-ylcarbonyl)-N-(3,4,5-trimethoxyphenyl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-2-amine
[0222]A solution of N-(3,4,5-Trimethoxyphenyl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-2-amine, Intermediate 1 (75 mg, 0.24 mmol) in CH2Cl2 (1.5 mL) was cooled to 0° C. and treated with benzoic anhydride (56.7 mg, 0.251 mmol) and pyridine (22 microliter, 0.273 mmol). After 1 hour the reaction mixture was diluted with CH2Cl2 and washed with water. The organic phase was collected, dried and concentrated. The residue was purified by silica gel chromatography (15-40% acetone / CH2Cl2). The product containing fractions were concentrated to give the title compound (75 mg).
example 2
[0223]
N-phenyl-6-(pyridin-3-ylcarbonyl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-2-amine
[0224]A solution of (2-chloro-7,8-dihydropyrido[4,3-d]pyrimidin-6(5H)-yl)(pyridin-3-yl)methanone, Intermediate 5 (0.025 g, 0.091 mmol) in isopropanol (0.1 mL) was treated with 3,4,5-trimethoxyaniline (0.021 g, 0.0498 mmol). The resulting mixture was heated to 60° C. and allowed to stir overnight. After being allowed to cool to room temperature EtOAc (10 mL) was added to the reaction mixture, and the resulting solution was then washed twice with brine (10 mL). The combined aqueous layers were washed once with EtOAc (10 mL). The organic layers were combined, then dried over anhydrous Na2SO4(s), filtered and concentrated in vacuo. The resulting crude residue was then purified over silica using a MeOH / CHCl3 gradient. The desired product was obtained in 54.8% yield (0.021 g, 0.049 mmol).
[0225]1H NMR (300 MHz, DMSO-d6) δ ppm 2.78-2.97 (m, 2H) 3.52-3.60 (m, 1H) 3.61 (s, 3H) 3.75 (s, 6H) 3.96 (m, 1H) 4.3...
example 3
2-anilino-N-pyridin-3-yl-7,8-dihydropyrido[4,3-d]pyrimidine-6(5H)-carboxamide
[0226]A solution of N-phenyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-2-amine, Intermediate 6 (75 mg, 0.33 mmol), 3-isocyanatopyridine (41.8 mg, 0.35 mmol) in CH2Cl2 (1.5 mL) was stirred at room temperature for 1 hour. After 1 hour the reaction mixture was diluted with CH2Cl2 and washed with sat. aqueous NaHCO3(aq). The organic phase was collected, dried and concentrated. The residue was dissolved in CH2Cl2 and washed with 3% aqueous LiCl solution and water. The organic phase was collected, dried and concentrated to give the title compound (45.9 mg).
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com