Preparation method for phenolic benzoxazine resin
A technology of benzoxazine and phenolic resin, which is applied in the field of preparation of phenolic benzoxazine, can solve the effects of benzoxazine resin storage stability, cured product heat resistance and flame retardancy, oxazine ring Issues such as low proportion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
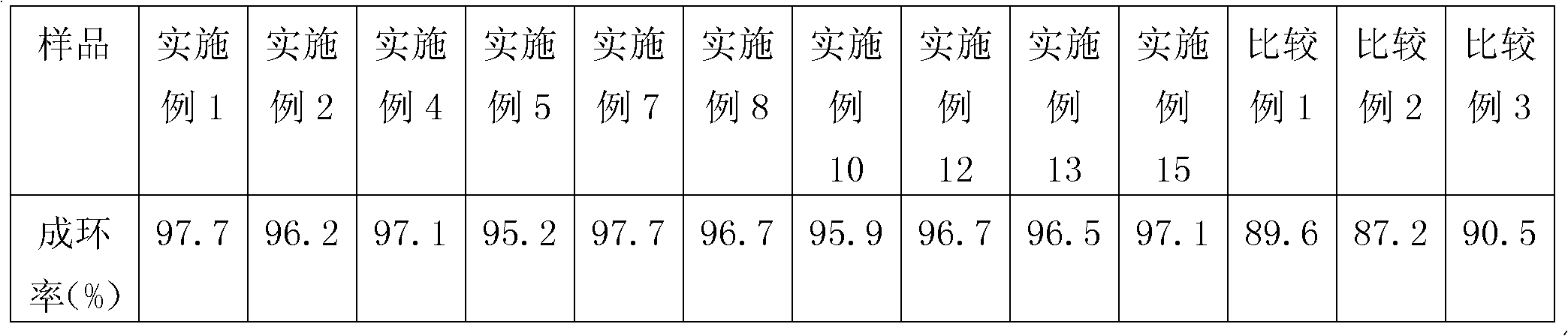
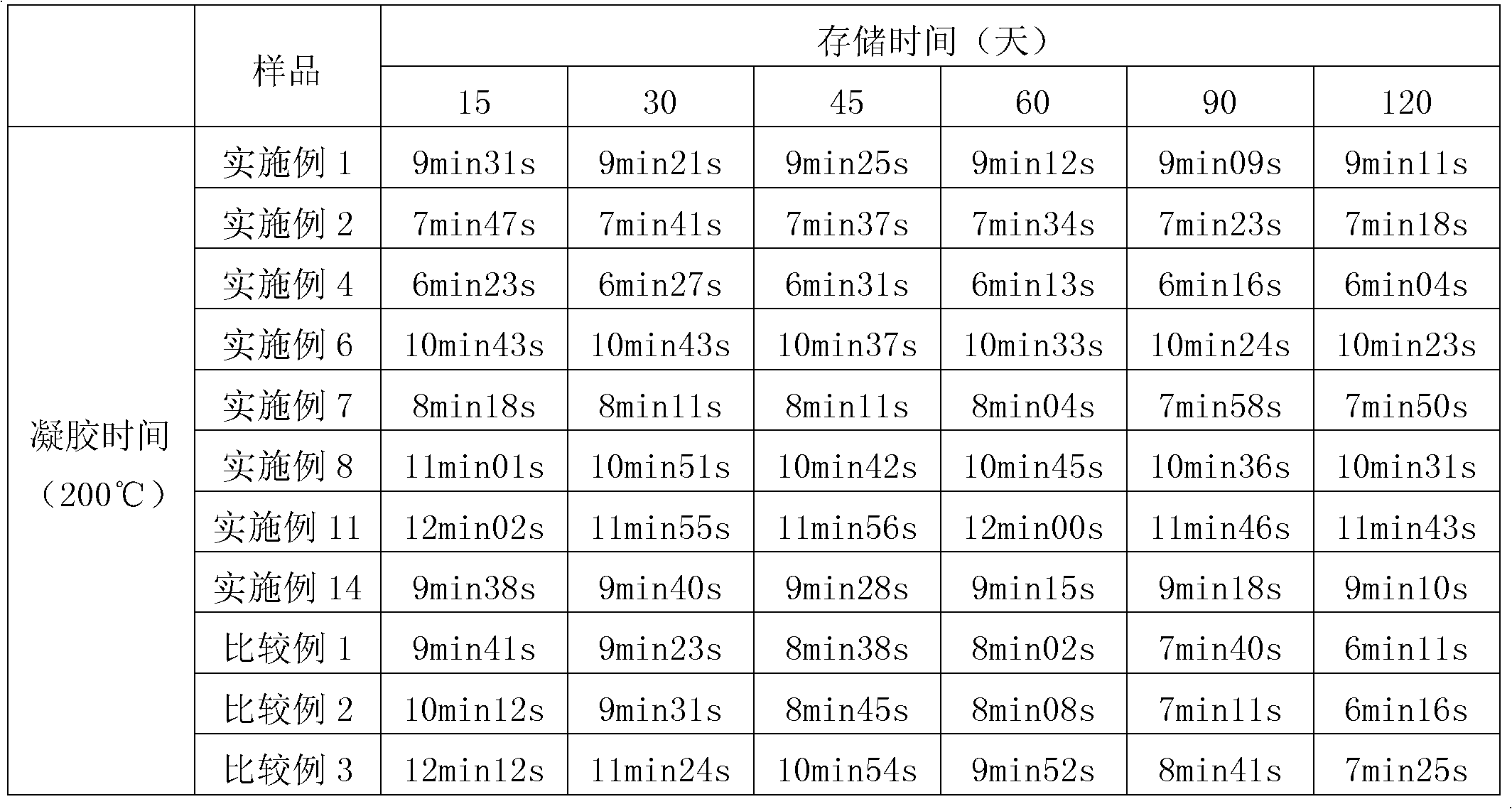
[0017] At room temperature, add 121 grams of aniline and 75 grams of paraformaldehyde into a reaction flask containing a water removal device, stir for 2 hours, then gradually raise the temperature to 60-90° C. and react for 5 hours. Then add 104 grams of thermoplastic phenolic resin (Novolak phenolic resin), and 2.4 grams of Zn (OAc) 2 2H 2 Add O in 3 times on average, raise the temperature to 80-100°C for 7 hours and remove water, then gradually raise the temperature to 100-130°C and keep the reaction for 2 hours to obtain benzoxazine resin, wash the benzoxazine resin with methanol and after drying, dissolve the benzoxazine resin with methyl ethyl ketone to obtain a novolak type benzoxazine resin solution. The resin cyclization rate is shown in Table 1, and the storage stability of the resin is shown in Table 2.
Embodiment 2
[0019] At room temperature, add 62 grams of allylamine and 226 grams of benzaldehyde into a reaction flask containing a water removal device, stir for 3.5 hours, then gradually raise the temperature to 60-90° C. and react for 3.5 hours. Then add 104 grams of thermoplastic phenolic resin (Novolak phenolic resin), and 2.8 grams of Mn(OAc) 2 4H 2 Add O in 3 times on average, raise the temperature to 80-100°C for 5.5 hours and remove water, then gradually raise the temperature to 100-130°C and keep the reaction for 3 hours to obtain benzoxazine resin, wash the benzoxazine resin with ethanol After drying, the benzoxazine resin is dissolved with 2-butanone to obtain a phenolic benzoxazine resin solution. The resin cyclization rate is shown in Table 1, and the storage stability of the resin is shown in Table 2.
Embodiment 3
[0021] At room temperature, add 118 grams of furfurylamine and 64 grams of paraformaldehyde into a reaction flask containing a water removal device, stir for 2.5 hours, then gradually raise the temperature to 60-90°C and react for 4 hours. Then add 104 grams of thermoplastic phenolic resin (Novolak phenolic resin), and 2.8 grams of Co(OAc) 2 4H 2 O and 1.1 g Ni(OAc) 2 2H 2 The mixture of O was added in 3 times on average, the temperature was raised to 80-100°C for 7 hours and the water was removed, and then the temperature was gradually raised to 100-130°C and kept for 2.5 hours to obtain benzoxazine resin. Wash the benzoxazine with n-propanol After the oxazine resin is dried, the benzoxazine resin is dissolved with toluene to obtain a phenolic benzoxazine resin solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com