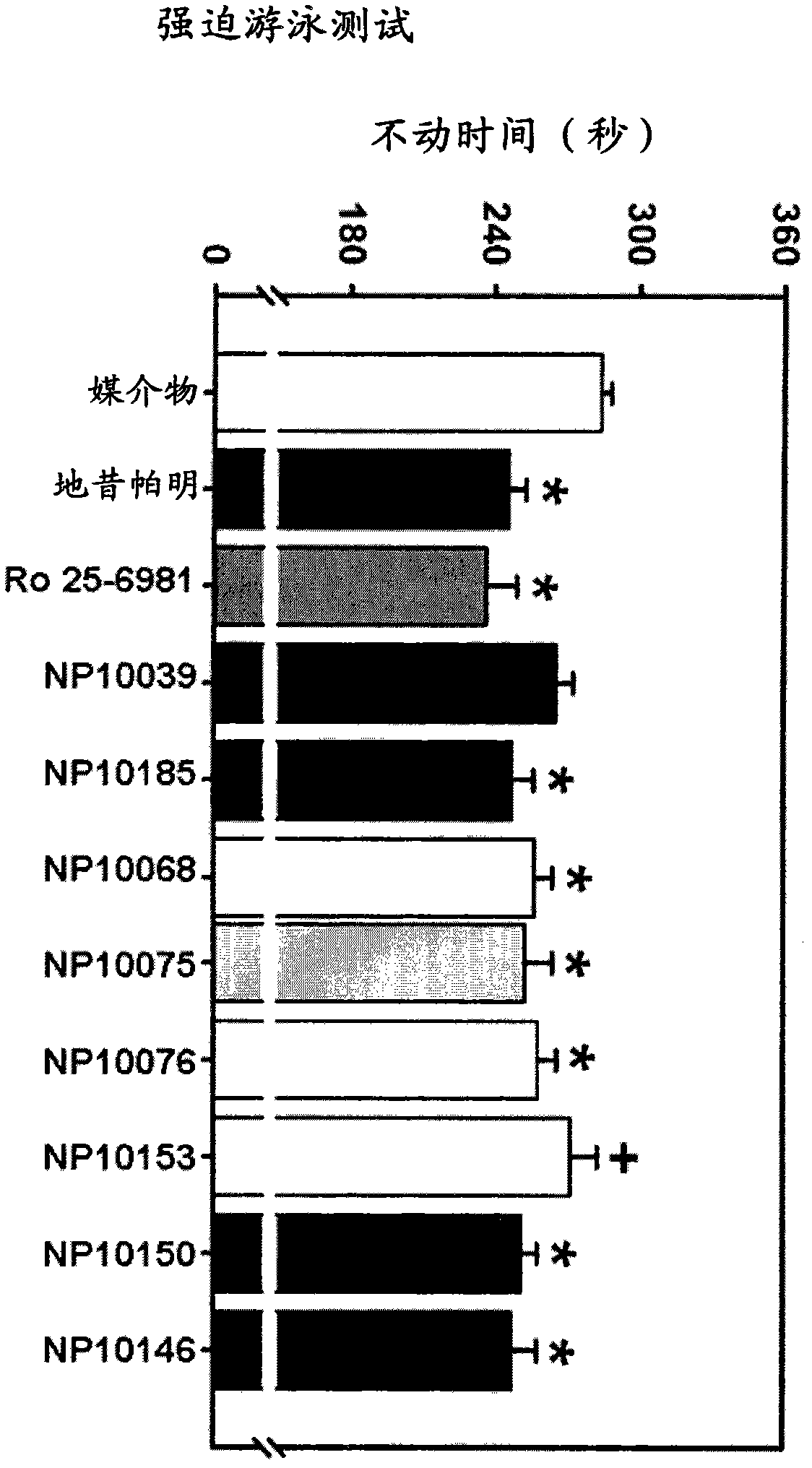
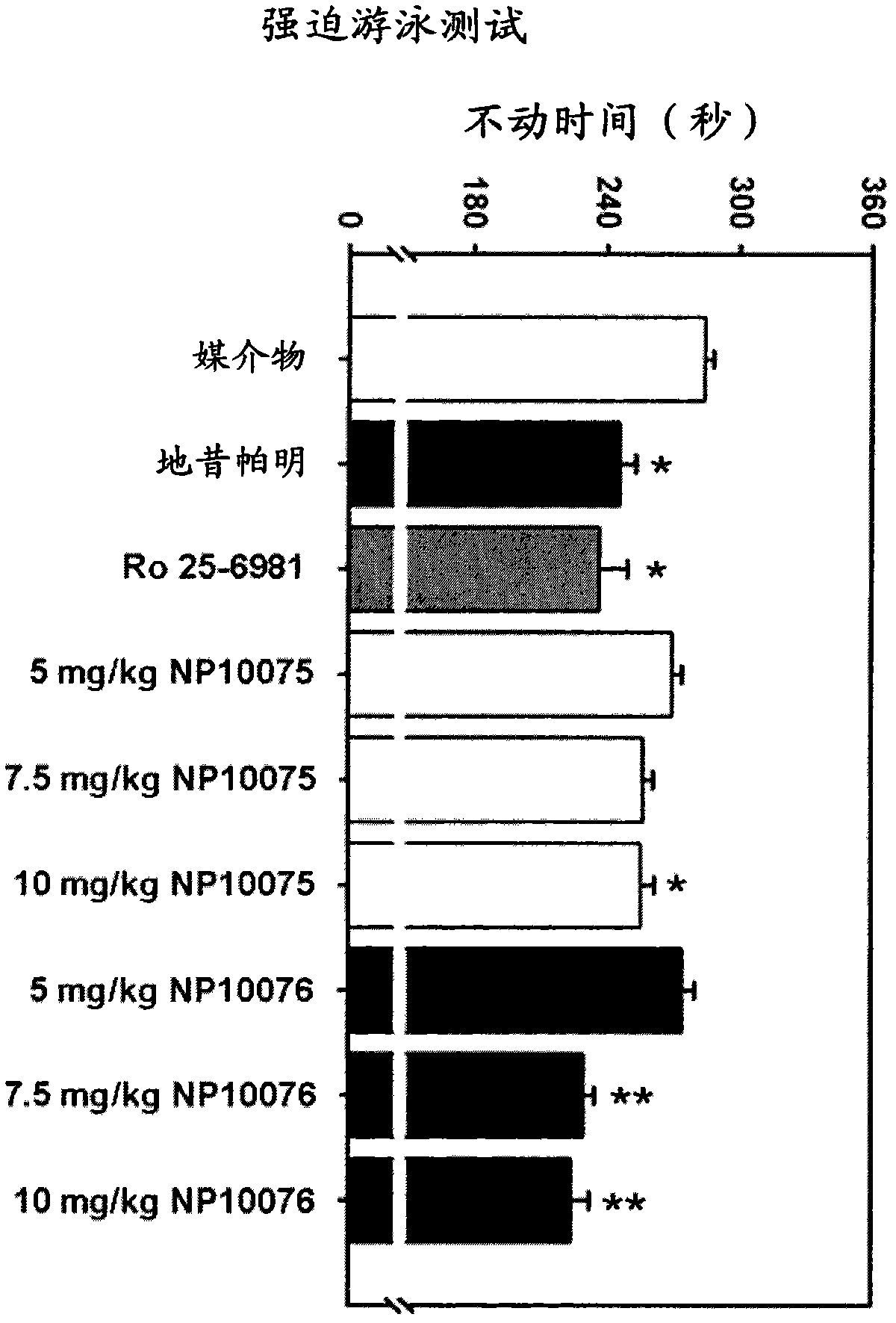
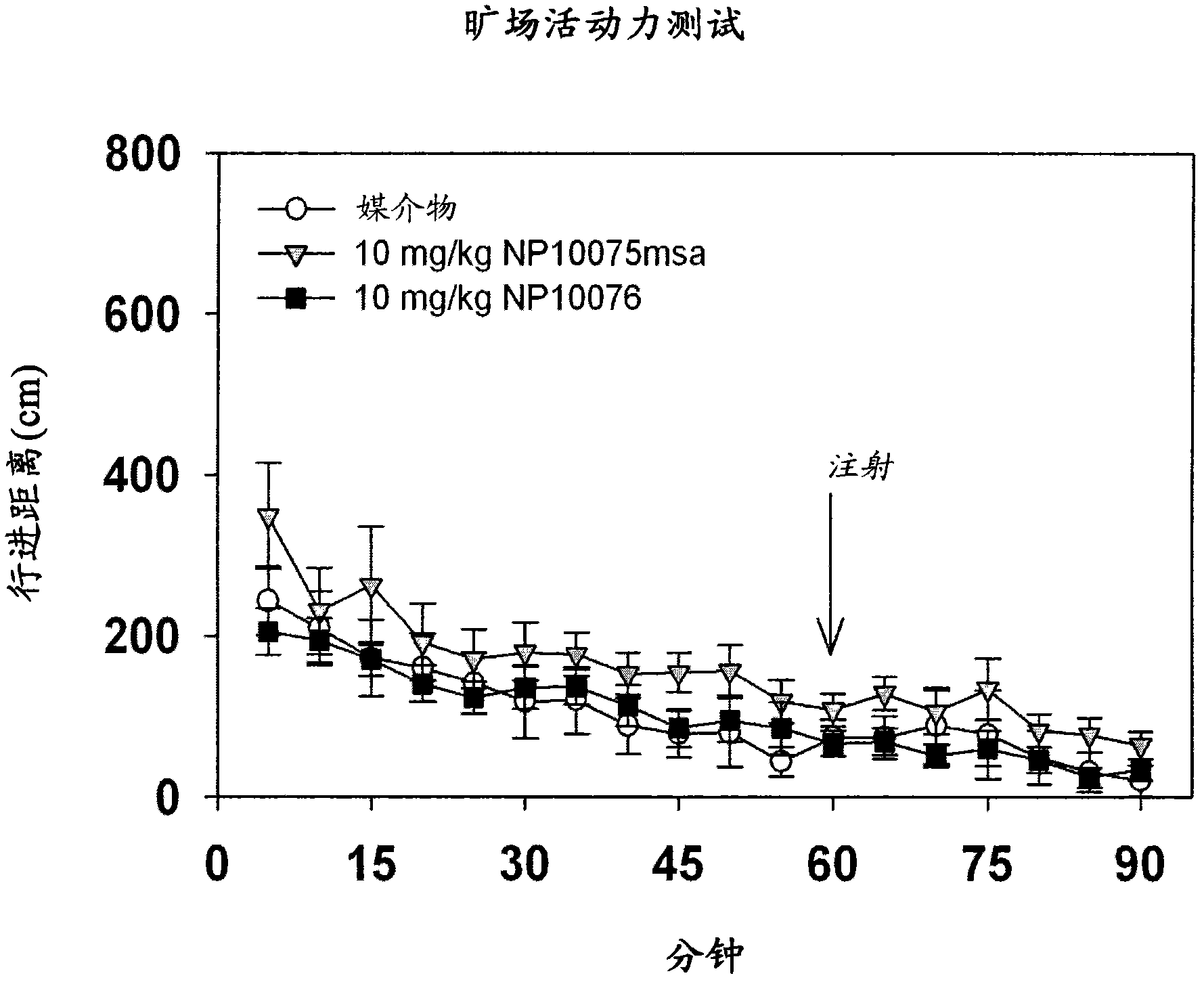
NMDA receptor antagonists for the treatment of neuropsychiatric disorders
A technology for mental disorders and nerves, applied in the field of NMDA receptor blockers, can solve problems such as hindering the use of NMDA receptor blockers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2
[0746] Examples 1 and 2. N-(4-{3-[4-(3,4-difluoro-phenyl)-piperazin-1-yl]-2-(S)-hydroxy-propoxy}- Phenyl)-methanesulfonamide (compound 1) and N-(4-{3-[2-(3,4-dichloro-phenylamino)-ethylamino]-2-(S)-hydroxy-propane oxy}-phenyl)-methanesulfonamide (compound 2).
[0747]
[0748]Step (i). 3-(4-Nitro-phenoxy)-2-(S)-propylene oxide (i-1). 4-Nitrophenol (6.6 mmol) was dissolved in 5 ml dry DMF. Cesium fluoride (19.9 mmol) was added to the reaction. The reaction mixture was stirred at room temperature for 1 hour and (S)-glycidyl m-nitrobenzenesulfonate (6.6 mmol) was added to the reaction mixture. The reaction was stirred at room temperature for 24 hours. Water (150 mL) was added and the solution was extracted with ethyl acetate. Dilute the organic phase in MgSO 4 dried and evaporated. The residue was purified by column chromatography using ethyl acetate:hexane (50:50) solvent system to give the desired product i-1. This step can be replaced by (R)-glycidyl m-nitrobenzenes...
Embodiment 3
[0756] Example 3.6-{3-[4-(4-chloro-phenyl)-piperazin-1-yl]-2-(S)-hydroxy-propoxy}-3H-benzoxazol-2-one (Compound 3).
[0757]
[0758] Step (i). 6-(2-(S)-oxiranylmethoxy)-3H-benzoxazol-2-one (ii-1). 5-Hydroxy-benzoxazole (310 mg) and cesium carbonate (780 mg) were combined in 6 mL of N,N-dimethylformamide. The reaction was stirred at room temperature for 1 hour. (S)-Glycidyl m-nitrobenzenesulfonate (520 mg) was added and the reaction was stirred overnight at room temperature. React with NH 4 Quenched with aqueous Cl and extracted with ethyl acetate. The organic layer was washed with NH 4 Cl aqueous solution and NaCl aqueous solution were washed, separated, and in Na 2 SO 4 Dry on top. Filtration, removal of solvent, and absorption onto silica gel. Elution with an ethyl acetate / methanol mixture (4:1) followed by removal of the solvent gave 445 mg of a yellow oily solid.
[0759] Step (ii). 6-{3-[4-(4-Chloro-phenyl)-piperazin-1-yl]-2-(S)-hydroxy-propoxy}-3H-benzoxazo...
Embodiment 4
[0763] Example 4. 4-{3-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-2-(S)-hydroxy-propoxy}-phenol (compound 4).
[0764]
[0765] Step (i). 3-(4-tert-Butyldimethylsilyloxy-phenoxy)-2-(S)-propylene oxide (iii-1). 4-(tert-Butyldimethylsiloxy)phenol (1.45 g, 6.25 mmol) in 5 ml dry THF was added dropwise to a suspension of NaH (0.158 g, 6.25 mmol) in 5 ml THF. After stirring at room temperature for 2 hours, glycidyl nitrobenzenesulfonate (1.30 g, 5 mmol) was added to the reaction mixture, and then 15-crown-5 (25 mol%) was added. After stirring for 24 hours, the reaction was poured into ice water and extracted with ethyl acetate. The organic phase was washed with water and brine, then dried over sodium sulfate and evaporated. The product was purified by column chromatography using EtOAc:hexane (1:9) (yield: 1.06 g 76%). 1 H NMR (400MHz, CDCl 3 )δ0.17 (6H, s), 0.98 (9H, s), 2.75 (1H, dd, J = 2.4, 4.4Hz), 2.89 (1H, q, J = 4.4Hz), 3.33-3.36 (1H, m ), 3.90 (1H, dd, J = 5.6, 10.8 Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com