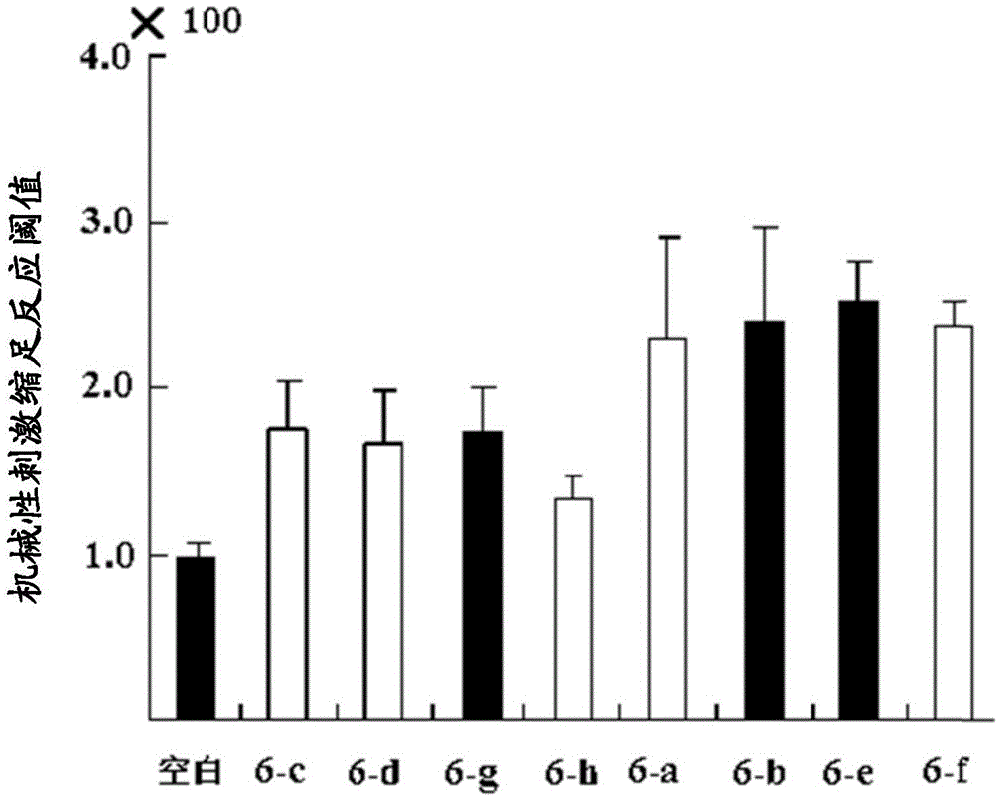
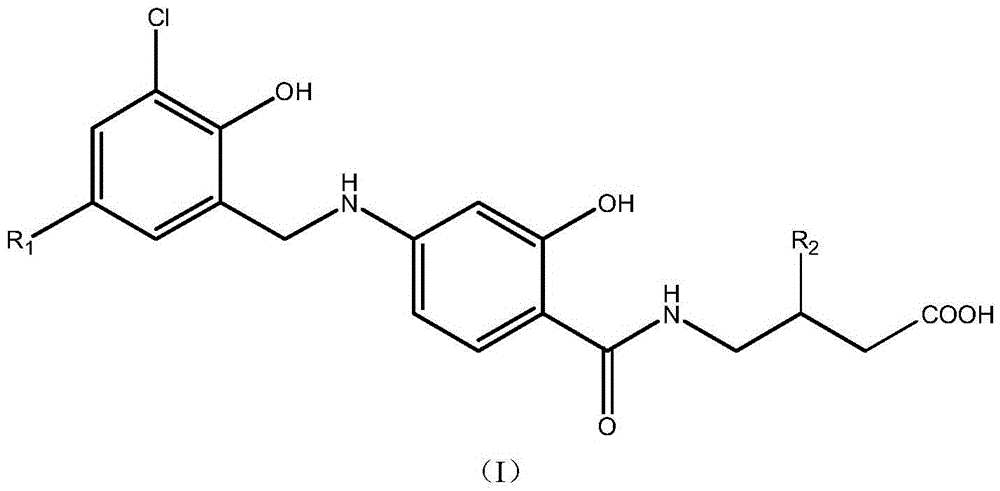
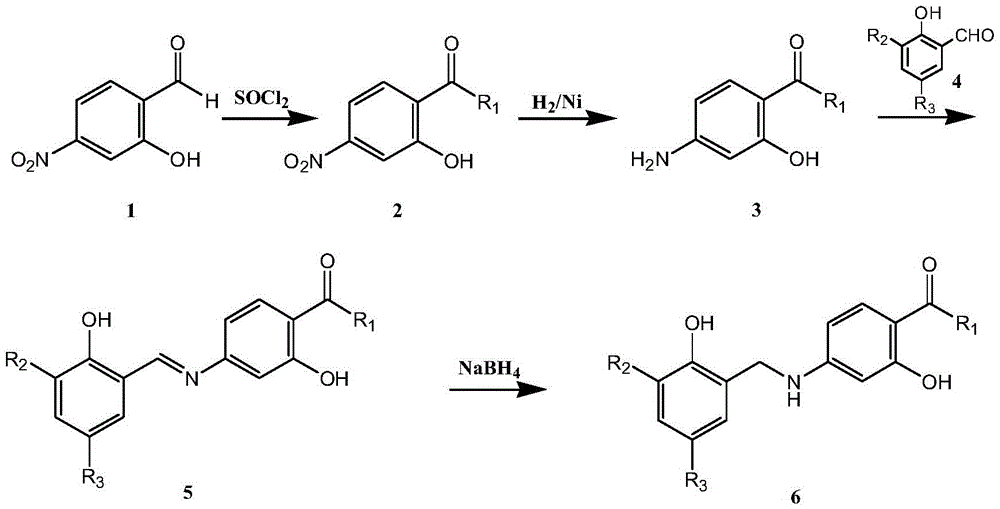
Amide derivatives of N-benzyl substituted aminosalicylic acid and 4-aminobutyric acid and their medicinal uses
A technology of aminosalicylic acid and amide derivatives, which is applied in the direction of carboxylic acid amide preparation, drug combination, medical preparations containing active ingredients, etc., which can solve the problems of unsatisfactory central nervous system distribution and achieve good neuropathic pain relief. pain effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 11-(2-Hydroxy-4-nitrobenzamidomethyl)-cyclohexylacetic acid (2-a)
[0022] Add 2g of 4-nitrosalicylic acid, 4g of thionyl chloride, 1-2 drops of pyridine, 20mL of dichloromethane into a 100mL eggplant-shaped bottle, react at 40°C for 12h, stop the reaction, and remove the solvent by rotary evaporation to obtain paranitrate Base salicyloyl chloride, add 15mL of dichloromethane for later use. Take another 100mL eggplant-shaped bottle, add 1.87g of gabapentin and 0.44g of sodium hydroxide, and dissolve with 15mL of water. At -5°C, add the freshly prepared p-nitrosalicyloyl chloride solution dropwise, the dropwise addition is completed in about 1 hour, react at 0°C for 2 hours, and react at 25°C for 12 hours, filter with suction, transfer the filtrate to a 50mL eggplant-shaped bottle, add concentrated hydrochloric acid Adjust the pH to 4-5, a large amount of white solid precipitated, filtered with suction, and dried. Silica gel column purification, eluent (petrole...
Embodiment 2
[0023] Example 23-(2-Hydroxy-4-nitrobenzoyl)aminomethyl-5-methylhexanoic acid (2-b)
[0024] Using 4-nitrosalicylic acid and pregabalin as raw materials, the rest of the steps are the same as compound 2-a. White solid, 38% yield. 1 HNMR500MHz, DMSO-d 6 ,δ(ppm): 0.83(d,3H,J=8.6Hz); 0.86(d,3H,J=8.6Hz); 1.10-1.25(m,2H); 1.65-1.73(m,1H); 2.12- 2.19(m,2H); 2.26-2.30(m,1H); 3.19-3.24(m,1H); 3.35-3.41(m,1H); 7.68(d,,1HJ=2.3Hz); 7.72(dd,1H , J=8.6Hz, J=2.3Hz); 8.04(d, 1H, J=8.6Hz); 8.88(t, 1H, J=5.5Hz); 12.08(s, 1H); 12.58(s, 1H)
Embodiment 3
[0025] Example 36-(2-Hydroxy-4-nitrobenzoyl)aminocaproic acid (2-c)
[0026] Using 4-nitrosalicylic acid and 6-aminocaproic acid as raw materials, the rest of the steps are the same as compound 2-a. White solid, 40% yield. 1 HNMR500MHz, DMSO-d 6 ,δ(ppm):1.30-1.37(m,2H); 1.51-1.59(m,4H); 2.22(t,2H,J=7.3Hz); 3.31(dd,2H,J=12.9Hz,J=6.8 Hz); 7.67(d,1H,J=2.3Hz); 7.71(dd,1H,J=8.7Hz,J=2.3Hz); 8.05(d,1H,J=8.7Hz); 8.92(t,1H, J=5.5Hz); 12.35(s,1H)
[0027] Example 41-{[2-hydroxyl-4-(2-hydroxyl-3,5-dichlorobenzyl)aminobenzoyl]aminomethyl}-cyclohexylacetic acid (6-a)
[0028] In a 500mL autoclave, add 150mL of methanol, 1g of 1-(2-hydroxy-4-nitrobenzamidomethyl)-cyclohexylacetic acid, 1g of Raney nickel, 3 times of hydrogen gas exchange, and react at 38°C for 12 Hour. The reaction was stopped, and the reaction liquid was rotary evaporated to 15 mL. A solution of 3,5-dichlorosalicylaldehyde (0.57 g) in methanol (5 mL) was added to the reaction liquid, and a large amount of solids were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com