Oxygen ether dual-triazole copper complex capable of catalyzing p-fluorophenylboronic acid and preparation method of oxygen ether dual-triazole copper complex
A technology of bistriazole copper and copper complexes, which is applied in the direction of organic chemical methods, copper organic compounds, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of expensive palladium catalysts, etc., and achieve simple and easy reaction operations OK, suitable for large-scale production, high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of 1-(4-(4-(1H-1,2,4-triazol-1-yl)phenoxy)phenyl)-1H-1,2,4-triazole (L)
[0029] Preparation of 1-(4-(4-(1H-1,2,4-triazol-1-yl)phenoxy)phenyl)-1H-1,2,4-triazole of the present invention Method, it is characterized in adopting " one pot method ", in DMF polar solvent, 1,1,2,2-tetrakis (4-bromophenyl) ethene, triazole, potassium carbonate and cupric oxide are under heating condition Prepare the organic compound; wherein the molar ratio of 4,4'-dibromodiphenyl ether: triazole: potassium carbonate: copper oxide is 2:10:30:1;
[0030]
[0031] The reaction temperature was 150°C, and the reaction time was 18 hours.
Embodiment 2
[0033] Cu(NO 3 ) 2 and the moles of 1-(4-(4-(1H-1,2,4-triazol-1-yl)phenoxy)phenyl)-1H-1,2,4-triazole (L) The ratio is 1:1;
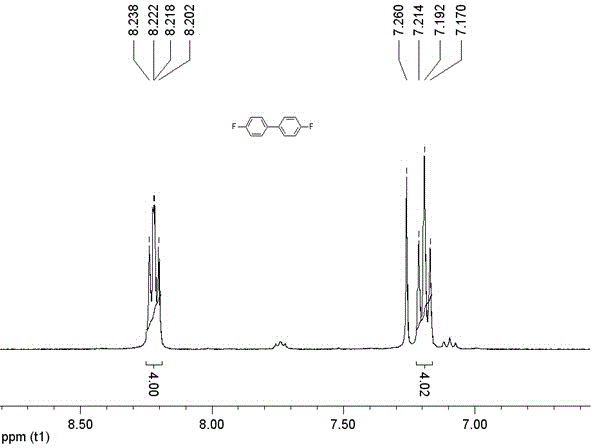
[0034] L (0.0304 g, 0.1 mmol) and Cu(NO 3 ) 2 (0.0242 g, 0.1 mmol) in H 2 O (6 mL) and CH 3OH (4 mL) was stirred in a mixed solvent at room temperature for half an hour and then filtered, and the filtrate was volatilized at room temperature as yellow rod-shaped crystals analyzed by X-ray single crystal diffraction. Yield: 45% (calculated based on L). Elemental analysis (C 32 h 24 CuN 14 o 8 ) Theoretical value (%): C, 48.27; H, 3.04; N, 24.63. Found: C, 48.25; H, 3.16; N, 24.66.
Embodiment 3
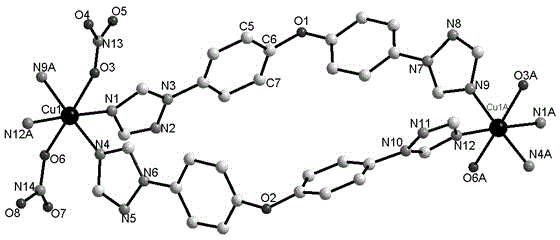
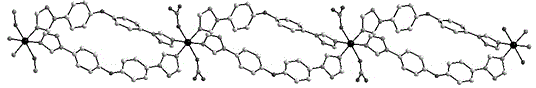
[0036] The crystal structure was determined using an APEX II CCD single crystal diffractometer, using graphite monochromatized Mokα rays (λ = 0.71073 ?) as the incident radiation, and ω -2 θ Diffraction points are collected by scanning, and the unit cell parameters are obtained by least square method correction. The crystal structure is solved by software from the difference Fourier electron density map, and corrected by Lorentz and polarization effects. All H atoms were synthesized by difference Fourier transform and determined by ideal position calculation. The detailed crystal determination data are shown in Table 1. Structural primitives see figure 1 , one-dimensional chain structure see figure 2 .
[0037] Table 1. Complexes 1 The crystallographic data of
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com