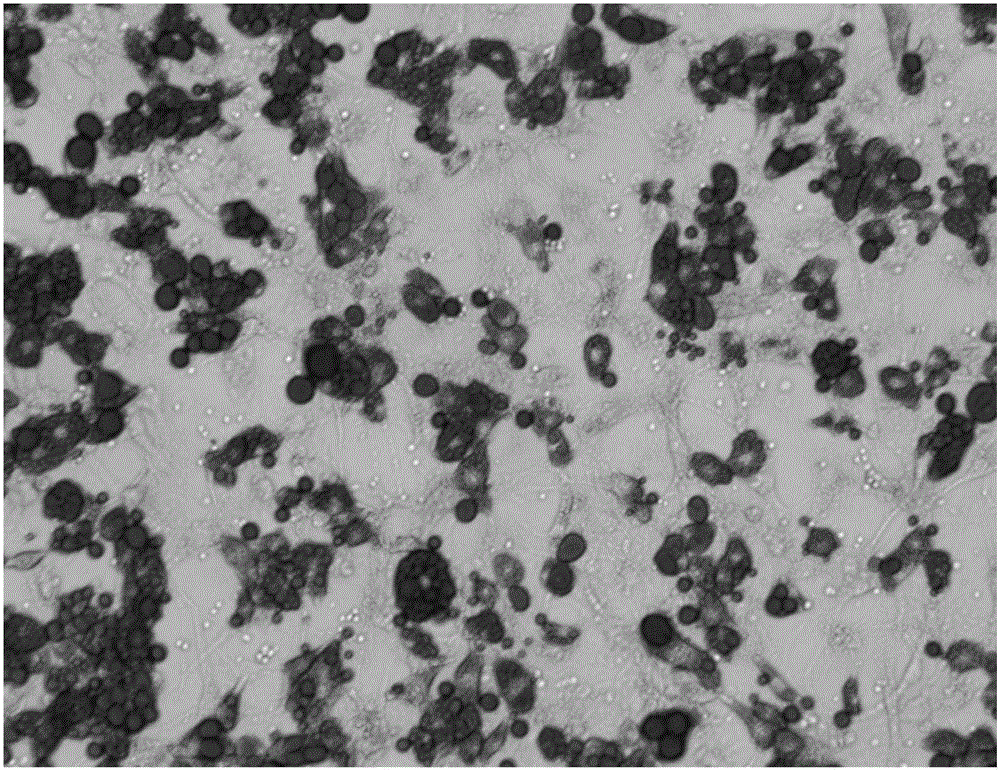
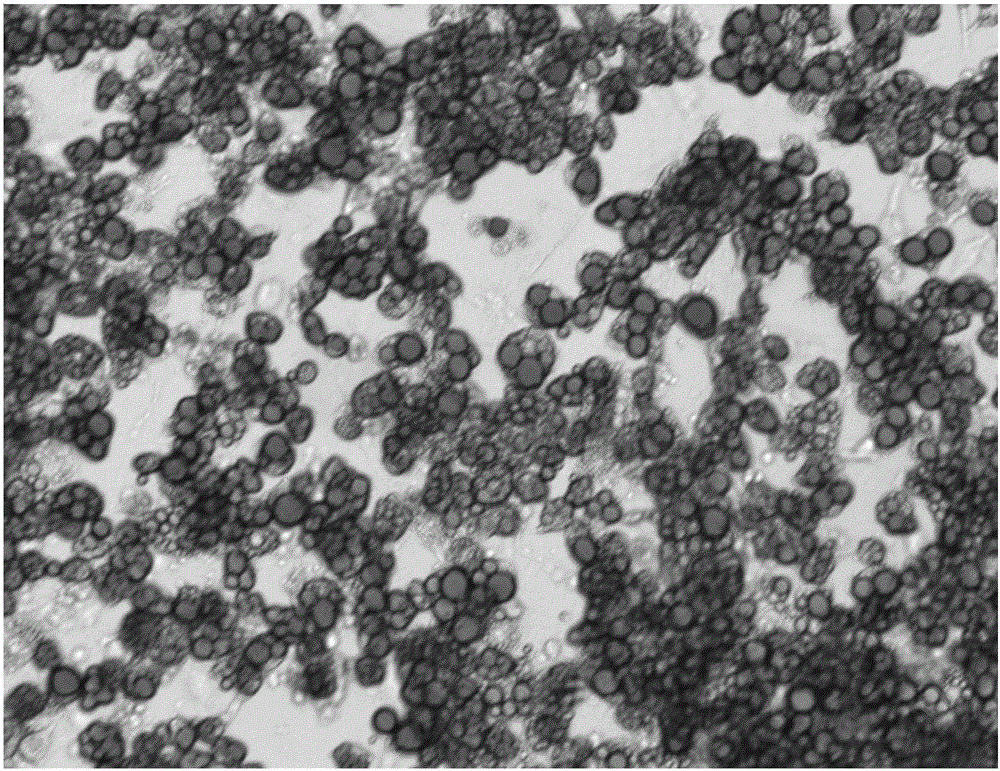
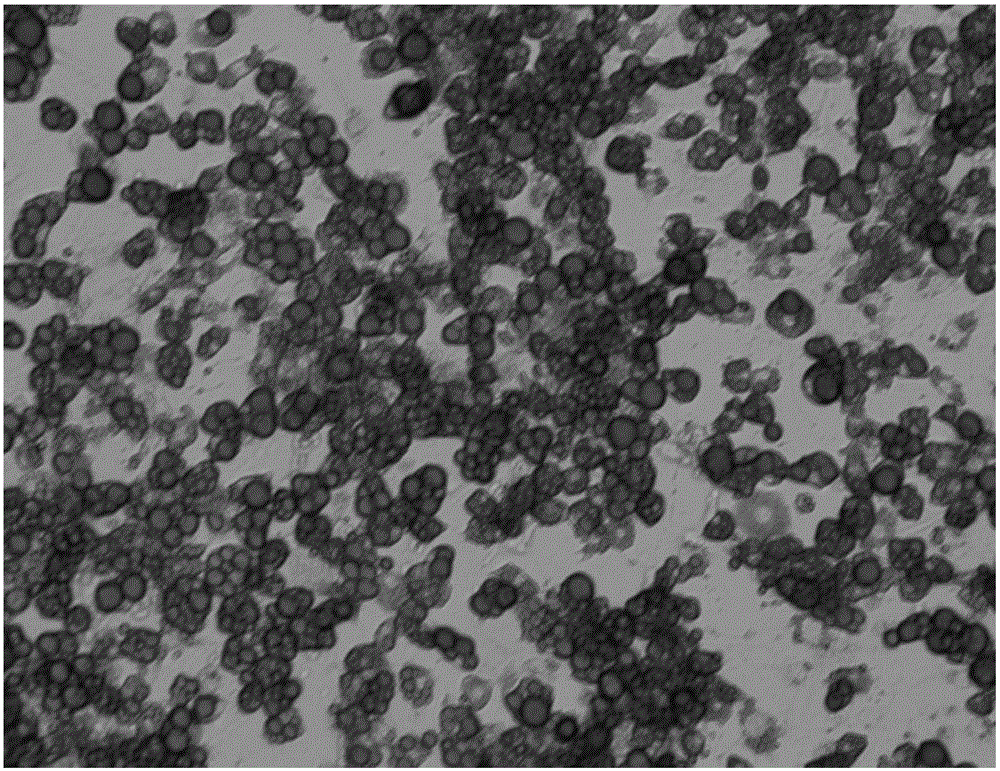
Induced differentiation method of 3T3-L1 preadipocytes line
A 3T3-L1, 1.3T3-L1 technology, applied in the field of induction and differentiation of 3T3-L1 preadipocyte line, can solve the problems of difficult to provide quantity for researchers, low conversion rate of adipocytes, long induction period, etc., to shorten the differentiation process, prolonged action time, stable conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Recovery of 3T3-L1 preadipocyte line: remove the cell line from liquid nitrogen, immediately place it in a 37±2°C water bath, take it out when the cell fluid is completely dissolved, centrifuge at 1000 rpm for 5 min at room temperature, discard the supernatant, and add to the culture The resuspended cells and culture medium were sucked into the culture dish, and the shape and number of cells were observed under the microscope, so that the cell suspension was 3×10 5 cells / ml into a culture vessel, which was then placed in 5% ± 1% CO 2 cultured in a constant temperature incubator at 37±2°C; wherein, the culture medium is a high-glucose DMEM medium containing 10% calf serum and 1% penicillin-streptomycin;
[0042]Cultivation of 3T3-L1 preadipocyte line: cells adhered under the microscope, shuttled and translucent, the cells were replaced with the culture medium every 3 days, the culture medium was tilted, the culture medium was removed with a pipette, and added along the w...
Embodiment 2
[0060] Recovery of 3T3-L1 preadipocyte line: remove the cell line from liquid nitrogen, immediately place it in a 37±2°C water bath, take it out when the cell fluid is completely dissolved, centrifuge at 1200 rpm for 3 min at room temperature, discard the supernatant, and add to the culture The resuspended cells and culture medium were sucked into the culture dish, and the cell morphology and number were observed under the microscope, so that the cell suspension was 6×10 5 cells / ml into a culture vessel, which was then placed in 5% ± 1% CO 2 cultured in a constant temperature incubator at 37±2°C; wherein, the culture medium is high-glucose DMEM medium containing 10% calf serum and 1.1% penicillin-streptomycin;
[0061] Cultivation of 3T3-L1 preadipocyte line: the cells adhered to the wall under the microscope, were shuttled and translucent, the cells were replaced with the culture medium every 2 days, the culture medium was tilted, the culture medium was removed with a pipette...
Embodiment 3
[0072] Recovery of 3T3-L1 preadipocyte line: remove the cell line from liquid nitrogen, immediately place it in a 37±2°C water bath, take it out when the cell fluid is completely dissolved, centrifuge at 1000 rpm for 5 min at room temperature, discard the supernatant, and add to the culture The resuspended cells and culture medium were sucked into the culture dish, and the shape and number of cells were observed under the microscope, and the cell suspension was 1×10 5 cells / ml into a culture vessel, which was then placed in 5% ± 1% CO 2 Cultured in a constant temperature incubator at 37±2°C; wherein, the culture medium is high-glucose DMEM medium containing 10% calf serum and 0.9% penicillin-streptomycin;
[0073] Cultivation of 3T3-L1 preadipocyte line: cells adhered under the microscope, shuttled and translucent, the cells were replaced with the culture medium every 3 days, the culture medium was tilted, the culture medium was removed with a pipette, and added along the wall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com