Functional targeting carrier material distearoylphosphatidylethanolamine-polyethylene glycol-polyethyleneimine compound and its modified liposome
A polymer, targeting technology, applied in liposome delivery, active ingredients of heterocyclic compounds, drug combination, etc., can solve problems such as strong cytotoxicity and application restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1, functional targeting carrier material DSPE-PEG shown in formula I 2000 -PEI 600 Synthesis and characterization of
[0076] 20 μmol PEI 600 (x is 0-15, y is 1-10) and compound DSPE-PEG shown in 20 μ mol formula III 2000 -NHS was dissolved in 4ml of anhydrous DMF, and the reaction solution mixture was gently stirred with a magnetic stirrer for 24 hours at room temperature under the protection of argon to carry out the substitution reaction. After the reaction was completed, the crude product was obtained, and then the crude product was transferred to the regenerated cellulose dialysis bag (MWCO 2500), dialyzed in deionized water for 48h to remove unreacted PEI and DMF solvent. Next, the reaction solution was freeze-dried to obtain the product represented by formula I as a dry white powder, which was stored at -20°C.
[0077] The synthetic route of this product is as follows figure 1 shown.
[0078] The reaction products were verified by matrix-assisted...
Embodiment 2
[0080] Embodiment 2, preparation and characterization of liposome
[0081] 1) Preparation of targeted blank liposomes modified by PEI
[0082] a. Precisely weigh lecithin (EPC), cholesterol (CHOL), DSPE-PEG 2000 -PEI 600 、DSPE-PEG 2000 Add an appropriate amount of chloroform to the eggplant-shaped bottle at a molar ratio of 63:32.5:0.5:4 to dissolve, then remove the organic reagent by rotary evaporation and drying under reduced pressure in a 40°C water bath at a speed of 40 rpm, and form a layer on the bottom and inner wall of the eggplant-shaped bottle Thin uniform lipid film;
[0083] b. Add an appropriate amount of 250mM ammonium sulfate solution to the lipid film obtained in step 1) for hydration: first ultrasonicate in a water bath for 5 minutes at room temperature, and the ultrasonic energy is 100W, until milky white uniform thick liposomes are formed, then transfer to JY92-IID type ultrasonic Further ultrasonication was carried out in the cell pulverizer (setting th...
Embodiment 3
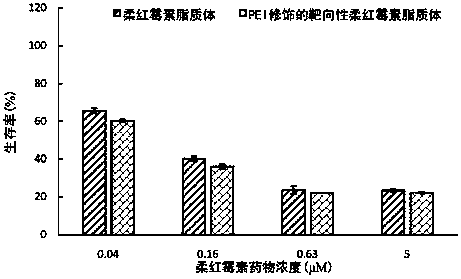
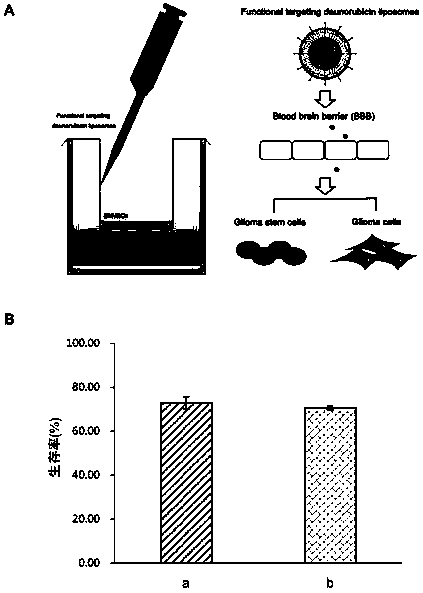
[0110] The drug effect experiment of the daunorubicin liposome of embodiment 3, PEI modification
[0111] (1) Cell uptake
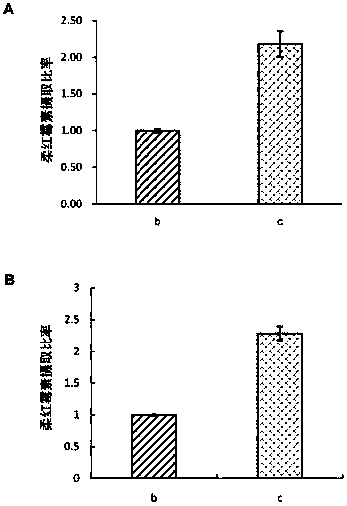
[0112] Figure 5 Shown are the fluorescence intensity results of cell uptake of glioma stem cells (A) and C6 glioma cells (B) after administration of each preparation group. The administration groups were daunorubicin liposome group (b) and PEI-modified daunorubicin liposome group (c). The corresponding values are represented in the histogram.
[0113] After administration and incubation for 4 hours, the uptake ratios of the daunorubicin liposome group and the PEI-modified daunorubicin liposome group obtained in step 2 of Example 1 in glioma stem cells were 1.00±0.03 and 2.18 respectively. ±0.17; the uptake ratios in C6 glioma cells were 1.00±0.01 and 2.28±0.11, respectively.
[0114] The results showed that the uptake ratio of C6 glioma cells and glioma stem cells to the PEI-modified daunorubicin liposome group obtained in step 2) of Example 1 was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com