Application of ASMT/CYP1A1 molecular signature in predicting clinical prognosis or immune characteristics of solid tumors
A technology of CYP1A1 and entities, which is applied in the field of application of ASMT/CYP1A1 molecular tags in predicting the clinical prognosis or immune characteristics of solid tumors, can solve the problems of no molecular tags, etc., and achieve the effect of improving the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention will be further described below in conjunction with specific experiments. It should be understood that the following content is only used to illustrate the present invention and is not intended to limit the protection scope of the present invention.
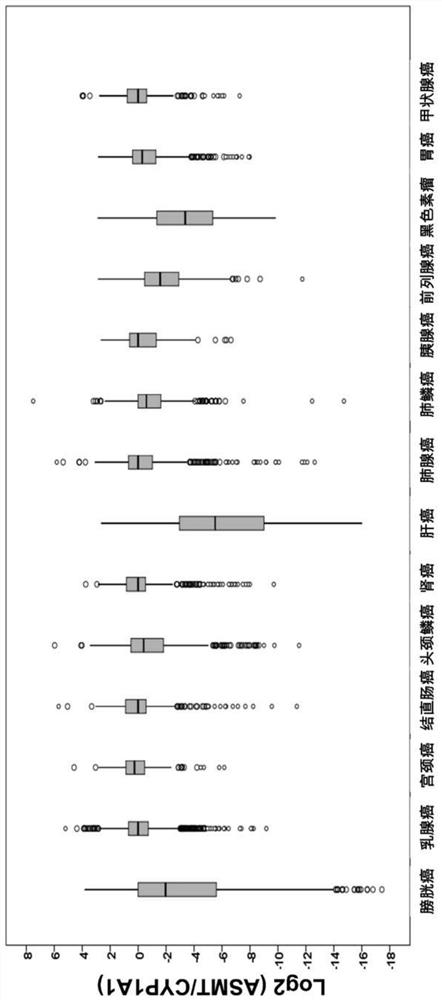
[0022] ASMT / CYP1A1 ratio in different solid tumors
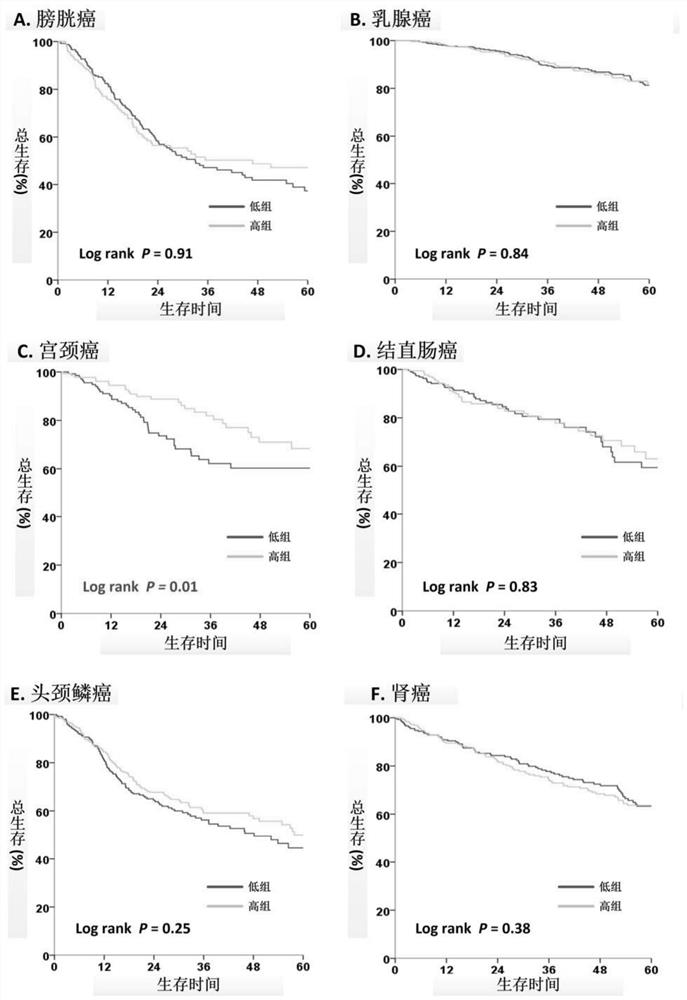
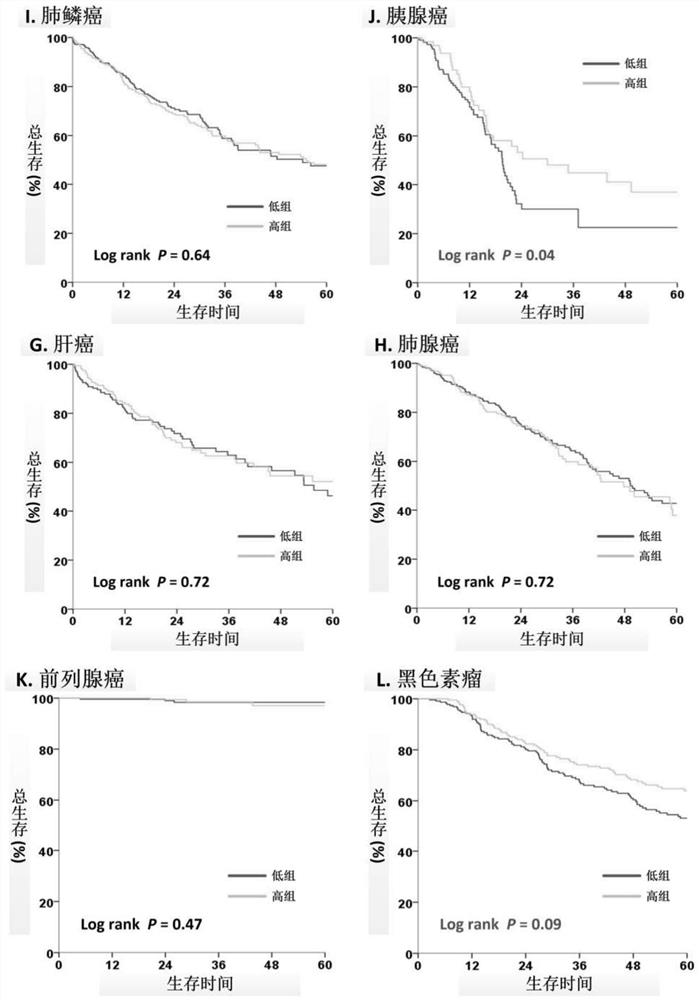
[0023] By analyzing 14 solid tumors (bladder cancer, breast cancer, cervical cancer, colon cancer, head and neck squamous cell carcinoma, kidney cancer, hepatocellular carcinoma, lung adenocarcinoma, lung squamous cell carcinoma, pancreatic cancer, prostate cancer, skin cancer) in the public database TCGA , gastric cancer and thyroid cancer) transcriptome and genome data of 6,658 tumor samples, and calculate the ratio of ASMT to CYP1A1 in each tumor tissue (ASMT / CYP1A1), which reflects the level of melatonin in the tumor tissue. The ASMT / CYP1A1 results of each tumor tissue were divided into High Index and Low Index ( figure 1 )Group.
[0024] Rela...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com