Preparation method of glimepiride crystal form I
A technology of glimepiride and crystal form, which is applied in the field of raw material drug preparation, can solve the problems that glimepiride is easy to degrade, deteriorate, change color, exceed the standard, and has no practical value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 (ethanol)
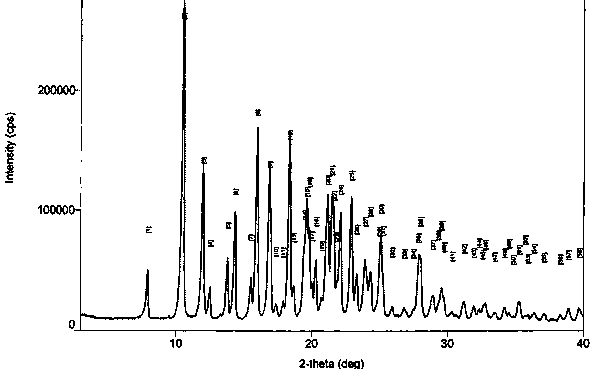
[0038] With 13.0g of crude glimepiride, add 130g of 95% ethanol, 4.18g of 27% ammonia water, and 25g of water, stir and dissolve, dissolve, filter, and slowly add 4.38g of glacial acetic acid (diluted with 26g of water) to the filtrate, dropwise After completion, continue to stir for 1 hour, filter, wash with water, and dry at 70°C to obtain 12.27g of the product, yield: 94.4%. It was determined to be crystal form II by XRD detection.
Embodiment 2
[0039] Example 2 (n-propanol)
[0040] Add 10.0g of glimepiride crude product, 100g of n-propanol, 3.21g of 27% ammonia water, and 20g of water, stir to dissolve, dissolve, filter, and slowly add 3.37g of glacial acetic acid (diluted with 20g of water) to the filtrate, dropwise After completion, continue to stir for 1 hour, filter, wash with water, and dry at 70°C to obtain 8.47g of the product with a yield of 84.7%. It was determined to be crystal form II by XRD detection.
Embodiment 3
[0041] Example 3 (isopropanol)
[0042] Add 5.0 g of crude glimepiride, 30 g of isopropanol, 10 g of purified water, and 1.61 g of 27% ammonia water, stir to dissolve, dissolve, filter, and slowly add 1.68 g of glacial acetic acid (diluted with 10 g of water) to the filtrate, dropwise After completion, continue to stir for 1 hour, filter, wash with water, and dry at 70°C to obtain 4.65 g of the product, yield: 93.0%. It was determined to be crystal form II by XRD detection.
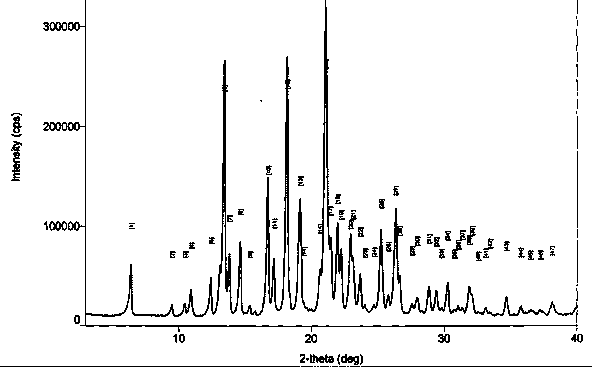
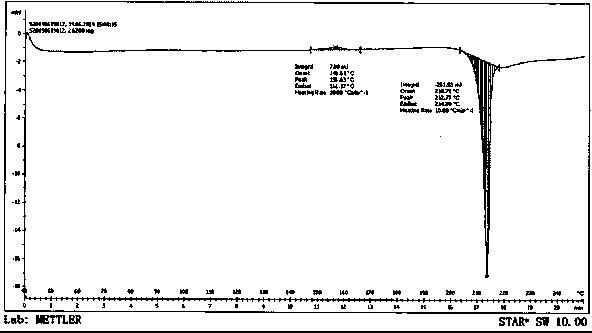
[0043] Preparation of crystal form I by transcrystallization
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com