Isoxazoline compound as well as preparation method and application thereof
A technology of isoxazolines and compounds, applied in the field of isoxazoline compounds and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
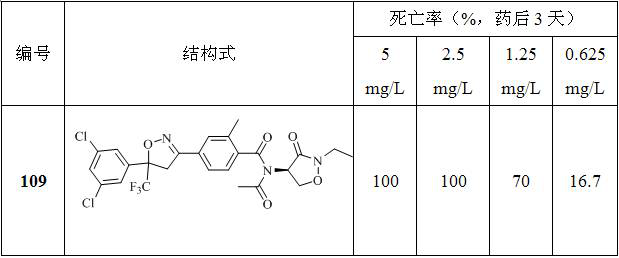
[0143] Embodiment 1: the preparation of compound 109
[0144]
[0145] Add 0.30 g (0.57 mmol) of 4-(5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl) to the reaction flask -N-((R)-2-ethyl-3-oxooxazolidin-4-yl)-2-methylbenzamide, 20 ml toluene and 0.11 g (1.09 mmol) triethylamine, then add 66.35 mg (0.85 mmol) of acetyl chloride was heated to reflux for reaction. After the reaction was monitored by TLC, water was added to the reaction solution and extracted with ethyl acetate. The organic phase was dried over anhydrous magnesium sulfate, filtered, concentrated, and purified by column chromatography to obtain 0.13 g of an oily substance.
[0146] The NMR and mass spectrometry data of compound 109 are as follows:
[0147] 1 H NMR (600 MHz, DMSO- d 6 ) δ 7.83 (t, 1H), 7.71 (s, 1H), 7.67 (dt, 1H),7.64 (d, 2H), 7.55 (d, 1H), 5.21 (t, 1H), 4.52 – 4.29 (m, 4H), 3.56 – 3.40(m, 2H), 2.40 (s, 3H), 2.15 (s, 3H), 1.13 (t, 3H). ESI-MS, m / Z: 594.23 [M+H+Na] + . ...
Embodiment 2
[0148] Embodiment 2: the preparation of compound 112
[0149]
[0150] Add 0.50 g (0.95 mmol) of 4-(5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl) to the reaction flask -N-((R)-2-ethyl-3-oxooxazolidin-4-yl)-2-methylbenzamide, 25 ml toluene and 0.19 g (1.88 mmol) triethylamine, then add 0.15 g (1.44 mmol) of cyclopropylformyl chloride was heated to reflux for reaction. After the reaction was monitored by TLC, water was added to the reaction solution and extracted with ethyl acetate. The organic phase was dried over anhydrous magnesium sulfate, filtered, concentrated, and purified by column chromatography to obtain 0.29 g of a yellow solid.
[0151] The NMR and mass spectrometry data of compound 112 are as follows:
[0152] 1 H NMR (600 MHz, Chloroform- d ) δ 7.63 (d, 1H), 7.60 (s, 1H), 7.56 – 7.49(m, 3H), 7.44 (t, 1H), 5.56 (t, 1H), 4.56 – 4.42 (m, 2H), 4.08 ( d, 1H), 3.75– 3.56 (m, 3H), 2.52 (s, 3H), 1.40 – 1.15 (m, 4H), 1.11 – 0.94 (m, 2H), 0.60(...
Embodiment 3
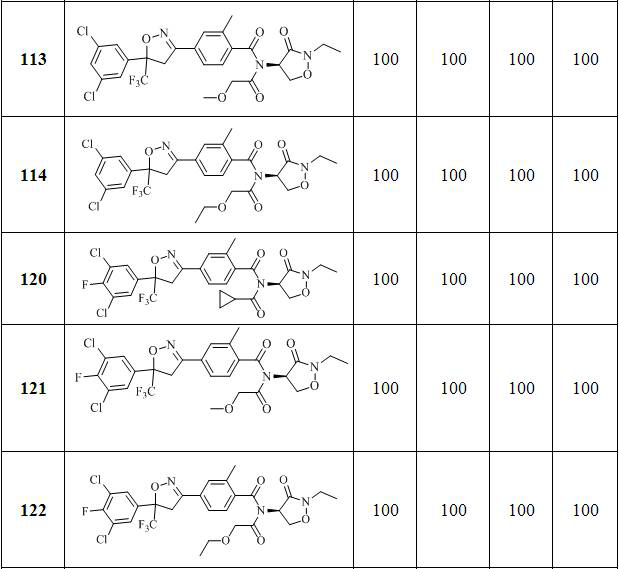
[0153] Embodiment 3: the preparation of compound 113
[0154]
[0155] Add 1.00 g (1.89 mmol) of 4-(5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl) to the reaction flask -N-((R)-2-ethyl-3-oxooxazolidin-4-yl)-2-methylbenzamide, 30 ml toluene and 0.38 g (3.76 mmol) triethylamine, then add 0.31 g (2.87 mmol) of methoxyacetyl chloride was heated to reflux for reaction. After the reaction was monitored by TLC, water was added to the reaction solution and extracted with ethyl acetate. The organic phase was dried over anhydrous magnesium sulfate, filtered, concentrated, and purified by column chromatography to obtain 0.53 g of an oily substance.
[0156] The NMR and mass spectrometry data of compound 113 are as follows:
[0157] 1 H NMR (600 MHz, DMSO- d 6) δ 7.82 (t, 1H), 7.72 (t, 1H), 7.68 – 7.65 (m,1H), 7.63 (d, 2H), 7.57 (dd, 1H), 5.08 (t, 1H), 4.49 – 4.28 ( m, 4H), 4.15 (s,2H), 3.57 – 3.36 (m, 2H), 3.22 (s, 3H), 2.39 (s, 3H), 1.12 (t, 3H). ESI-MS,m / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com