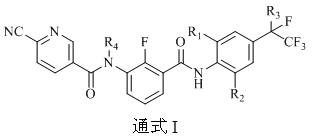
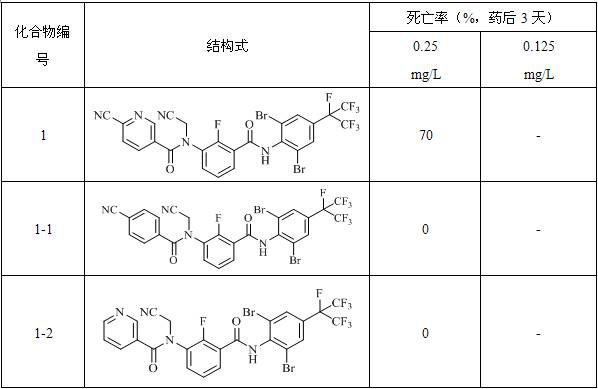
Amide compound and application thereof
A technology of amide compounds and compounds, which is applied in the field of compounds, can solve the problems of unreported insecticidal activity and achieve excellent insecticidal effects and suitable control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: Preparation of Intermediate II.1
[0096]
[0097] To 30 mL of DMF was added 1.00 g (1.80 mmol) of N-(2,6-dibromo-4-heptafluoroisopropylphenyl)-2-fluoro-3-aminobenzamide (Intermediate III-1 , prepared with reference to the method reported in WO2011093415 or WO2010018714), 0.37 g (2.68 mmol) of potassium carbonate, 0.27 g (1.80 mmol) of sodium iodide and 0.26 g (2.19 mmol) of bromoacetonitrile, heated to 100 degrees for reaction. After the reaction was monitored by TLC, water and ethyl acetate were added for extraction, the organic phase was precipitated under reduced pressure, and the residue was purified by column chromatography to obtain 0.50 g of a white solid, namely intermediate II.1. The NMR and mass spectrometry data of Intermediate II.1 are as follows:
[0098] 1 H NMR (600 MHz, Chloroform- d ) δ 8.11 (d, 1H), 7.88 (s, 2H), 7.64 – 7.58(m, 1H), 7.29 (t, 1H), 7.05 (td, 1H), 4.52 -4.44(br, 1H), 4.24 ( d, 2H). LC-MS(m / z, ESI): 594.01(M+H) + .
Embodiment 2
[0099] Example 2: Preparation of Intermediate II.2
[0100]
[0101]
[0102] Add 10 grams of N-(2-bromo-6-iodo-4-heptafluoroisopropylphenyl)-2-fluoro-3-nitrobenzamide (obtained with reference to the method reported in CN109206335A), 15 grams of anhydrous Stannous chloride, 200 ml of 1,4-dioxane and 8 ml of concentrated hydrochloric acid were heated to 60°C and stirred for reaction. After the reaction was monitored by TLC, the organic solvent was distilled off under reduced pressure. Add 500 ml of ethyl acetate, and add an appropriate amount of saturated aqueous sodium hydroxide solution to adjust the pH to 10. After stirring thoroughly, use diatomaceous earth to filter out the precipitated insoluble matter. After the filtrate is extracted with ethyl acetate and water, the organic layer is washed with anhydrous sulfuric acid. Dried over magnesium, filtered and concentrated under reduced pressure gave a beige solid, the crude product was purified by column chromatography...
Embodiment 3
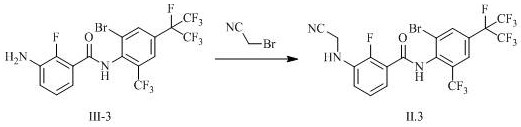
[0105] Example 3: Preparation of Intermediate II.3
[0106]
[0107] To 60 mL of DMF was added 2.00 g (3.67 mmol) of N-(2-bromo-6-trifluoromethyl-4-heptafluoroisopropylphenyl)-2-fluoro-3-aminobenzamide (middle Body III-3, prepared with reference to the method reported in WO2011093415 or WO2010018714), 0.76 gram (5.50 mmol) potassium carbonate, 0.56 gram (3.74 mmol) sodium iodide and 0.53 gram (4.42 mmol) bromoacetonitrile, heated to 100 degree of response. After the completion of the reaction as monitored by TLC, water and ethyl acetate were added for extraction, the organic phase was precipitated under reduced pressure, and the residue was purified by column chromatography to obtain 0.78 g of a white solid, namely intermediate II.3. The NMR and mass spectrometry data of Intermediate II.3 are as follows:
[0108] 1 H NMR (600 MHz, Chloroform- d ) δ 8.18 (d, 1H), 8.16 – 8.13 (m, 1H), 7.94– 7.90 (m, 1H), 7.63 – 7.55 (m, 1H), 7.29 (t, 1H), 7.05 (td, 1H), 4.52 – 4.45(m, 1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com