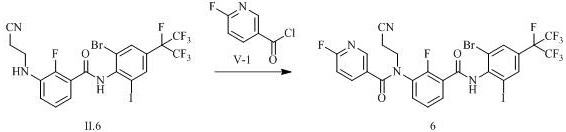
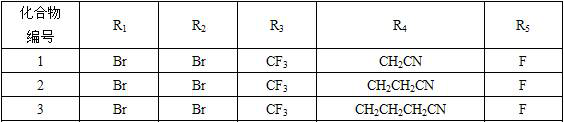
Isophthalamide compound and application thereof
A technology of isophthalamide and compound, which is applied in the field of isophthalamide compounds, and can solve problems such as unreported insecticidal activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164]Example 1: Preparation of intermediate compound II.1
[0165]
[0166]To 30 ml of DMF was added 1.00 g (1.80 mmol) N-(2,6-dibromo-4-heptafluoroisopropylphenyl)-2-fluoro-3-aminobenzamide (Intermediate IV-1 , Prepared by referring to the method reported in WO2011093415 or WO2010018714), 0.37 g (2.68 mmol) of potassium carbonate, 0.27 g (1.80 mmol) of sodium iodide and 0.26 g (2.19 mmol) of bromoacetonitrile, heated to 100 degrees for reaction. After the reaction was monitored by TLC, water and ethyl acetate were added for extraction, the organic phase was desolvated under reduced pressure, and the residue was purified by column chromatography to obtain 0.50 g of a white solid, that is, intermediate II.1. The NMR and MS data of Intermediate II.1 are as follows:
[0167]1H NMR (600 MHz, Chloroform-d) δ 8.11 (d, 1H), 7.88 (s, 2H), 7.64-7.58(m, 1H), 7.29 (t, 1H), 7.05 (td, 1H), 4.52 -4.44(br, 1H), 4.24 ( d, 2H). LC-MS(m / z, ESI): 594.01(M+H)+.
Embodiment 2
[0168]Example 2: Preparation of intermediate compound II.2.
[0169]
[0170]To 50 ml of DMF was added 1.50 g (2.70 mmol) N-(2,6-dibromo-4-heptafluoroisopropylphenyl)-2-fluoro-3-aminobenzamide (Intermediate IV-1 ), 0.56 g (4.05 mmol) of potassium carbonate, 0.41 g (2.74 mmol) of sodium iodide and 0.43 g (3.21 mmol) of bromopropionitrile, the temperature is raised to 100 degrees for reaction. After the reaction was monitored by TLC, water and ethyl acetate were added for extraction, the organic phase was desolvated under reduced pressure, and the residue was purified by column chromatography to obtain 0.25 g of a white solid, namely Intermediate II.2. The NMR and MS data of Intermediate II.2 are as follows:
[0171]1H NMR (600 MHz, Chloroform-d) δ 8.16 (d, 1H), 7.87 (s, 2H), 7.52 – 7.46(m, 1H), 7.20 (t, 1H), 6.90 (td, 1H), 4.46-4.40 (m, 1H), 3.66 – 3.60 (m,2H), 2.72 (t, 2H). LC-MS(m / z, ESI): 608.01(M+H)+.
Embodiment 3
[0172]Example 3: Preparation of Intermediate Compound II.3
[0173]
[0174]To 30 ml of DMF was added 1.00 g (1.80 mmol) N-(2,6-dibromo-4-heptafluoroisopropylphenyl)-2-fluoro-3-aminobenzamide (Intermediate IV-1 ), 0.37 g (2.68 mmol) of potassium carbonate, 0.27 g (1.80 mmol) of sodium iodide and 0.32 g (2.16 mmol) of bromobutyronitrile, heated to 100 degrees for reaction. After the reaction was monitored by TLC, water and ethyl acetate were added for extraction, the organic phase was desolventized under reduced pressure, and the residue was purified by column chromatography to obtain 0.12 g of white solid, which is intermediate II.3. The NMR and MS data of Intermediate II.3 are as follows:
[0175]1H NMR (600 MHz, Chloroform-d) δ 8.16 (d, 1H), 7.87 (s, 2H), 7.46-7.42 (m, 1H), 7.18 (t, 1H), 6.94 (td, 1H), 4.17-4.10 (m, 1H), 3.43 ( q, 2H), 2.54(t, 2H), 2.08-2.02 (m, 2H). LC-MS(m / z, ESI): 622.03(M+H)+.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com