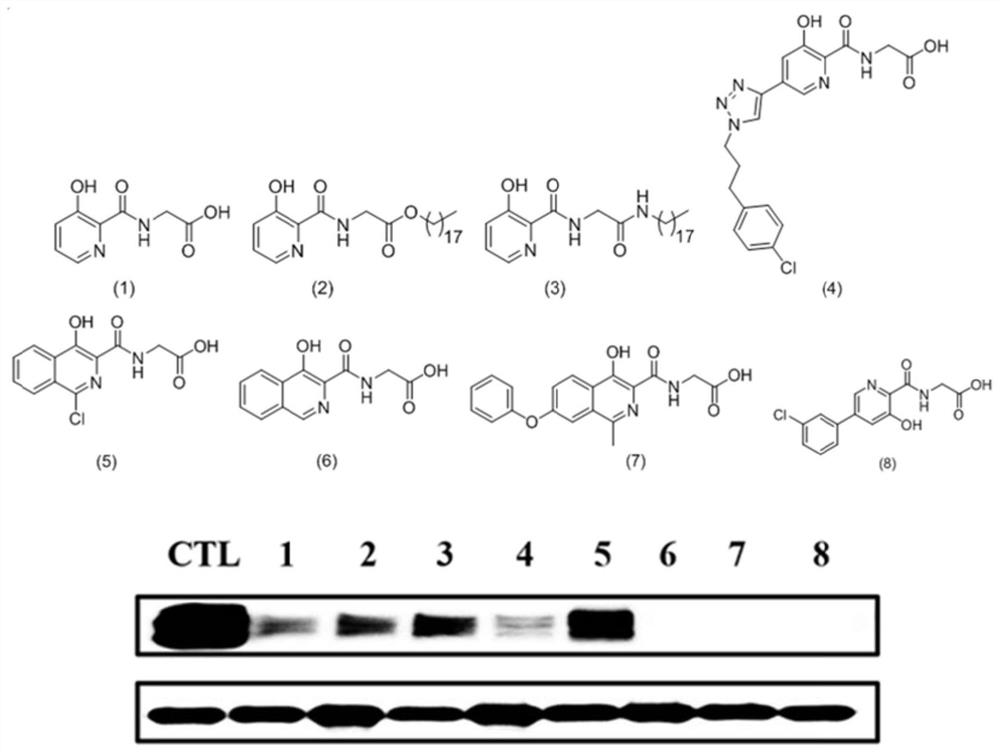
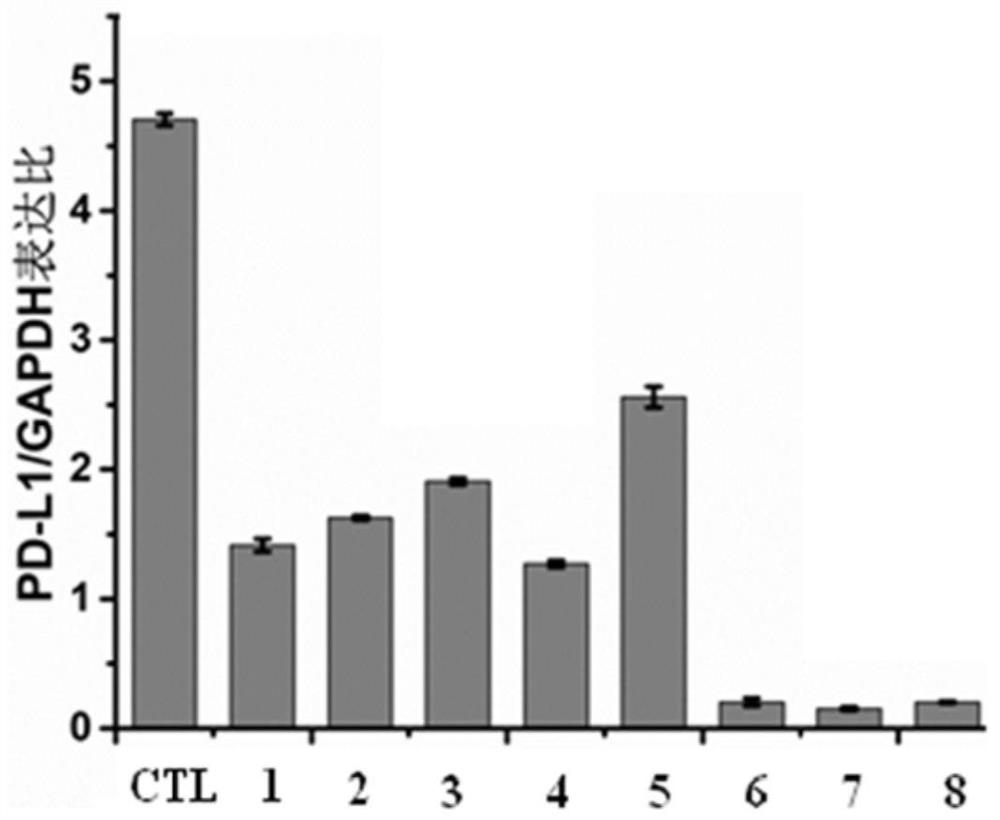
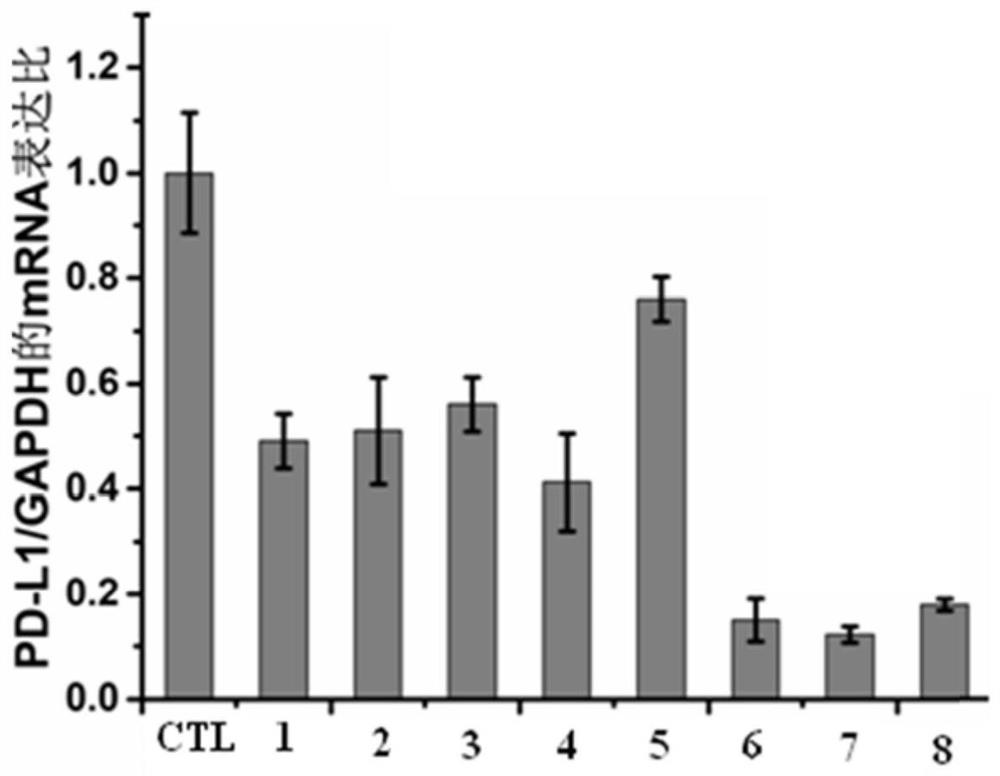
Anti-tumor drug sensitizer based on N-(3-hydroxypyridine-2-carbonyl) glycine and application thereof
A technology of anti-tumor drugs and sensitizers, applied in the field of medicine, can solve the problems of high risk of side effects, high price, inconvenient preparation and storage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1: The preparation route of the esterified derivative of N-(3-hydroxypyridine-2-carbonyl)glycine is as follows.
[0111] R 1 for-O-C 1-20 Alkyl or -O-C 6-12 Aryl.
[0112] Take compound (2) as an example.
[0113]
[0114] Compound (1) (200 mg, 1.02 mmol) was dissolved in 20 mL of dry N,N dimethylformamide, and oleyl alcohol (329 mg, 1.22 mmol) and 4-dimethylaminopyridine (6.23 mg, 0.05 mmol) were added. N 2 Protected and added dicyclohexylcarbodiimide (252 mg, 1.22 mmol) dropwise under ice bath, stirred for 1 h, then removed the ice bath and continued to react overnight. After the reaction was completed, the solvent was removed by rotary evaporation under reduced pressure, and the compound (2) was obtained as a white solid with a yield of 86.9%. Formula: [C 26 h 45 N 3 o 3 ] + , Calc. 448.33, found 448.23.
Embodiment 2
[0115] Example 2: The preparation route of amidated derivatives of N-(3-hydroxypyridine-2-carbonyl)glycine is as follows.
[0116] R 1 for NH 2 or -NH-C 1-20 .
[0117] Take compound (3) as an example.
[0118]
[0119] Compound (1) (200 mg, 1.02 mmol) was dissolved in 20 mL of dry N, N dimethylformamide, and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (603 mg , 3.06mmol), 1-hydroxybenzotriazole (207mg, 1.53mmol) and oleylamine (328mg, 1.23mmol), N 2 Protect and add N,N-diisopropylethylamine (527 mg, 4.08 mmol) dropwise under ice bath, stir for 1 h, then remove the ice bath and continue the reaction overnight. After the reaction was completed, most of the solvent was removed by rotary evaporation under reduced pressure, and ethyl acetate was redissolved, followed by washing with 1N hydrochloric acid and saturated brine, drying and concentrating, and using n-hexane:ethyl acetate=3:1 as the mobile phase for column purification. After drying, compound (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap