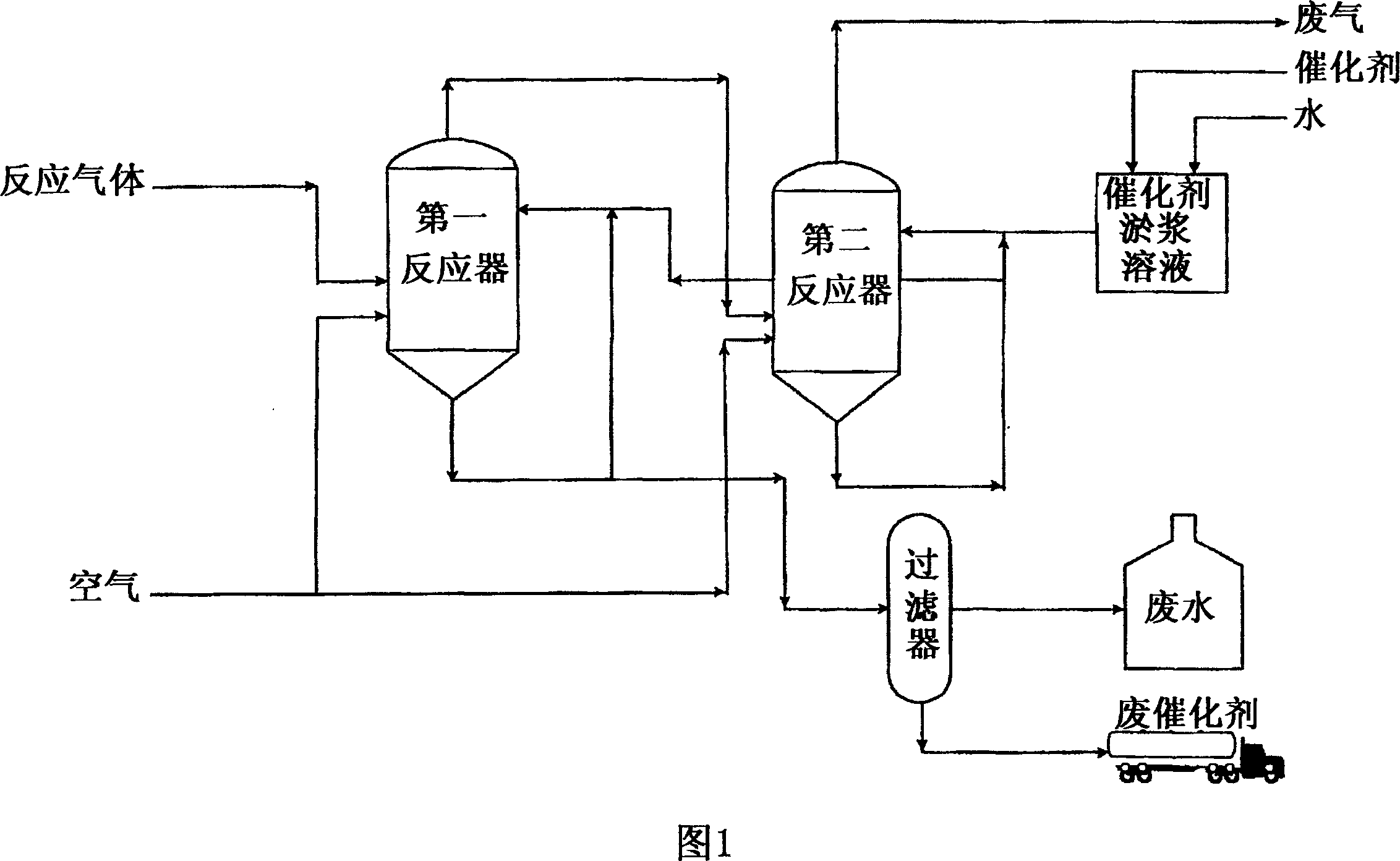
Desulfurization for simultaneous removal of hydrogen sulfide and sulfur dioxide
A technology for sulfur dioxide and hydrogen sulfide, applied in chemical instruments and methods, separation methods, separation of dispersed particles, etc., can solve problems such as low treatment efficiency, environmental problems, excessive loss of chemicals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 illustrates the effect of simultaneous removal of hydrogen sulfide and sulfur dioxide in a batch wet oxidation reaction without using a catalyst.
[0051] 1.5 liters of water was introduced into a stirred reactor, where hydrogen sulfide, sulfur dioxide and air were introduced through a porous diffuser located at the bottom of the reactor so that the flow rate of hydrogen sulfide was 10ml / min, and the flow rate of sulfur dioxide was 5ml / min. The air flow rate is 100ml / min. After the reaction, the product gas was analyzed by gas chromatography, and the result was converted into removal efficiency using the following equation.
[0052]
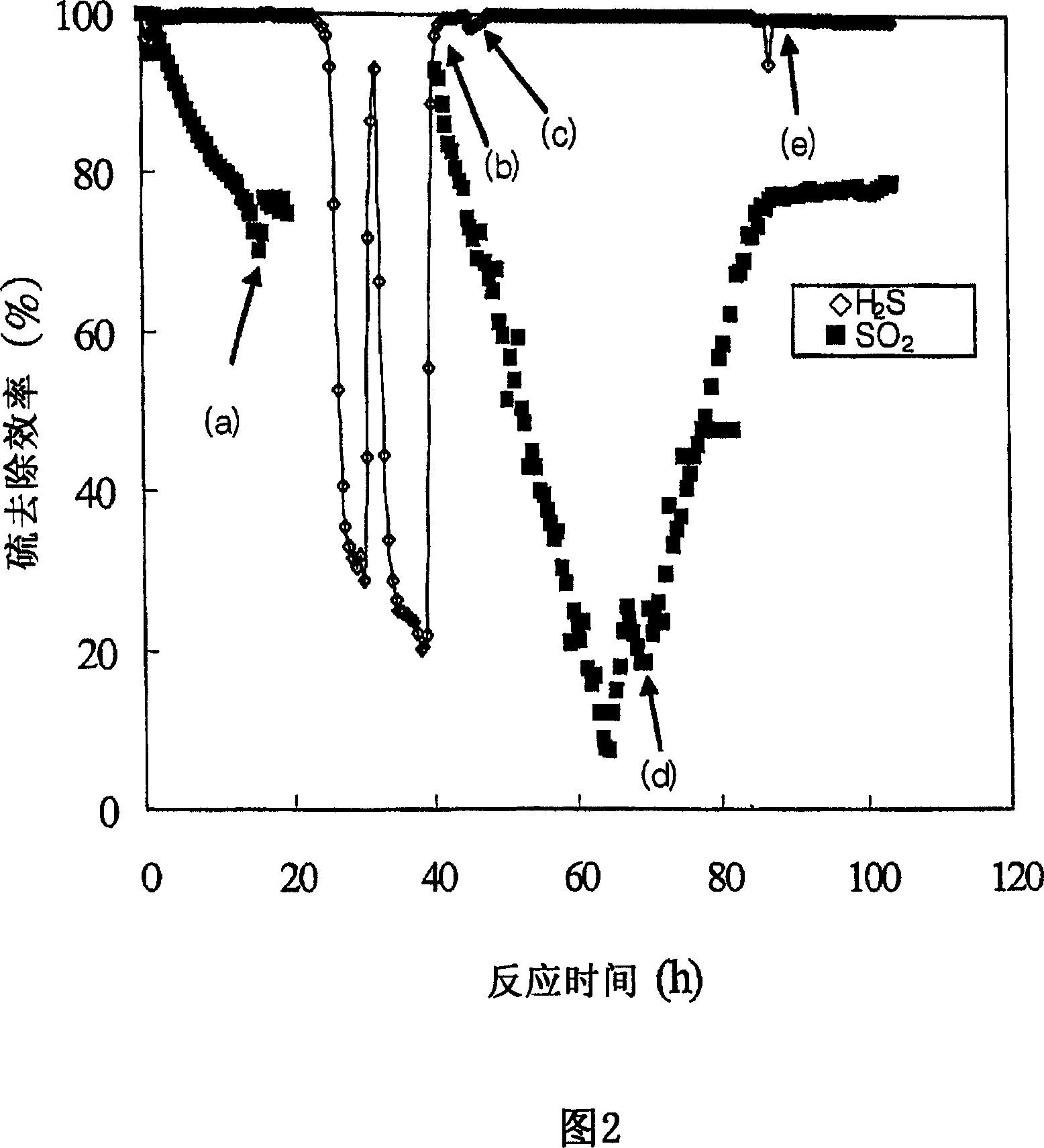
[0053] Figure 2 shows the sulfur removal efficiency as a function of reaction time. In Fig. 2, (a), (b), (c), (d) and (e) respectively represent when SO 2 Closed, SO 2 Open, H 2 S is off, H 2 S opens and the point when the oxidant becomes oxygen.
[0054] As shown in Figure 2, it can be seen that the removal efficiency of hydrogen ...
Embodiment 2
[0056] The procedure of Example 1 was repeated except that 3 g of 6 wt% Fe / MgO catalyst was used in Example 2. Disperse 20g of MgO in 200ml of water, and then add 1N ferric nitrate solution to it to make Fe relative to MgO 6wt%, and then dry and calcinate the result at 450°C to prepare 6wt% Fe / MgO catalyst.
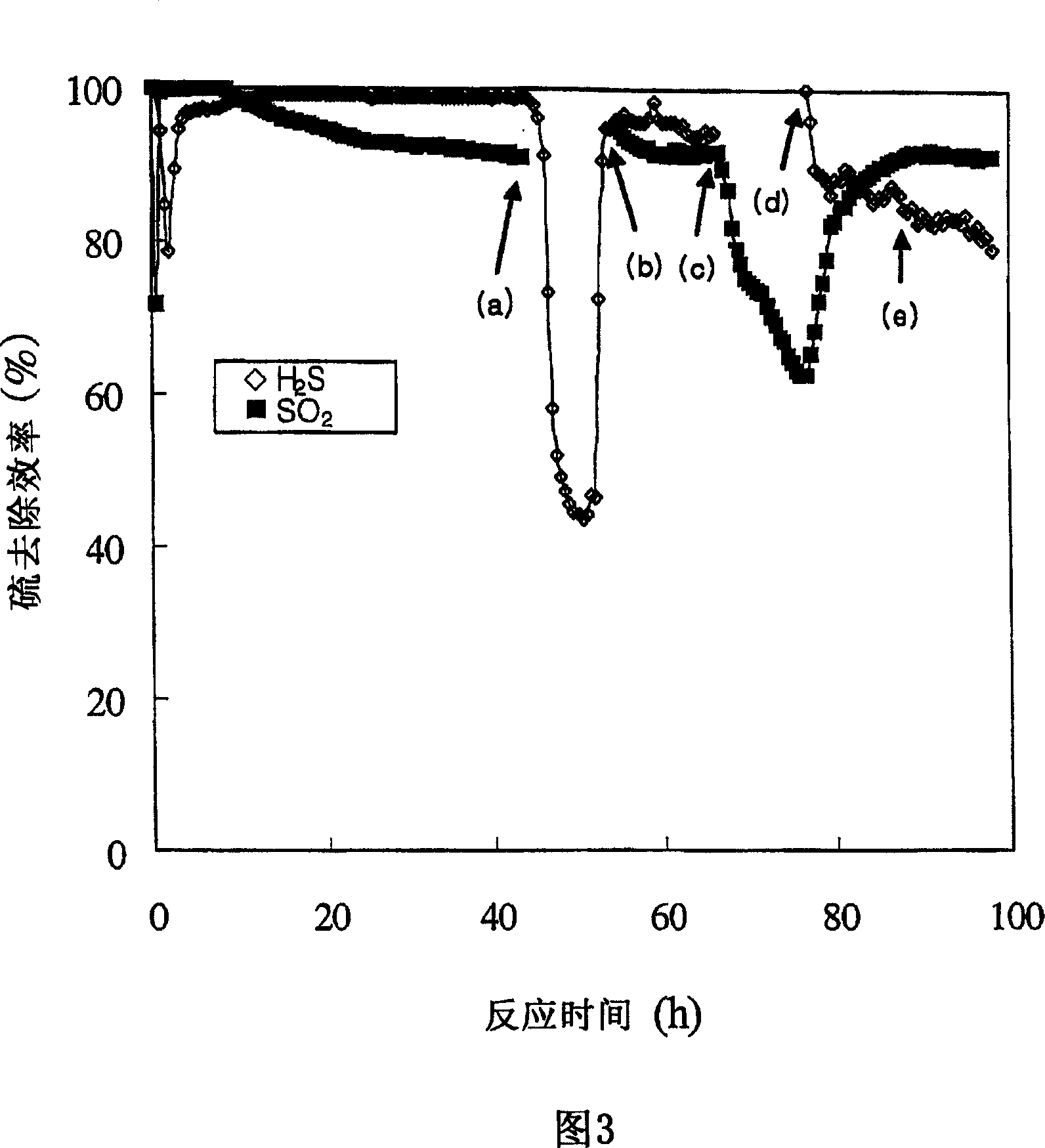
[0057] The results are shown in Figure 3. In Fig. 3, (a), (b), (c), (d) and (e) respectively represent when SO 2 Closed, SO 2 Open, H 2 S is off, H 2 S turns on and the point when the oxidant becomes nitrogen.
[0058] Fig. 3 shows that the respective removal efficiency and simultaneous removal efficiency of hydrogen sulfide and sulfur dioxide in Example 2 using Fe / MgO catalyst are much higher than that in Example 1 where no catalyst is used. After point (e) when the oxidant becomes nitrogen, the removal efficiency of hydrogen sulfide and sulfur dioxide gradually decreases.
Embodiment 3
[0060] Using 3g of 6wt% Fe / MgO catalyst, except that the reaction gas ratio between hydrogen sulfide and sulfur dioxide becomes 5:5, 5:15, and 10:5, and except that air is used as the oxidant, the total gas flow rate is fixed at 110 Except for / ml / min, the same desulfurization step as in Example 2 was repeated.
[0061] As a result, when the concentration of sulfur dioxide is higher than that of hydrogen sulfide, the removal efficiency of hydrogen sulfide is high, as shown in FIG. 4. This means that sulfur dioxide plays a key role as an oxidant in the desulfurization reaction of the present invention. Specifically, when the ratio between hydrogen sulfide and sulfur dioxide is 5:5 or 10:5, a sudden drop in removal efficiency is observed at the beginning of the reaction, which indicates that there is an induction period in the desulfurization reaction of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com