Liposome-containing radiographic contrast medium and preparation method thereof
A technology for radiography and contrast agents, which is applied in the preparation of X-ray contrast agents, liposome delivery, and medical preparations with inactive ingredients, etc., and can solve problems such as failure to achieve results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
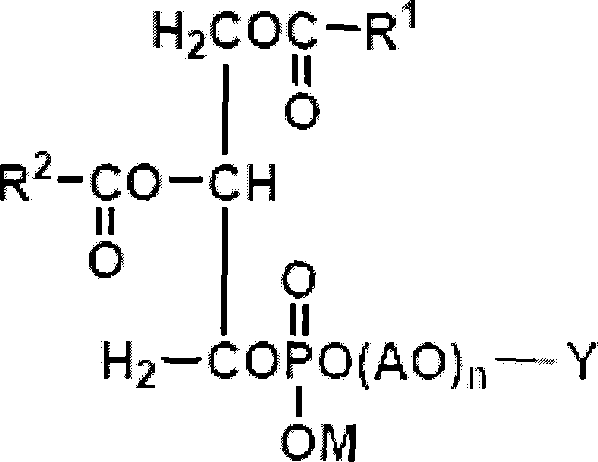
Image
Examples
Embodiment 1
[0114] Determination of iodine in iodine compounds
[0115] The sample (liposome dispersion) was dialyzed with isotonic saline solution. After the dialysis, ethanol was added therein to destroy the liposomes, and then the amount of iodine compound encapsulated in the liposomes was measured by absorptiometry. The ratio of the above-mentioned amount of iodine compound to the total amount of iodine compound in the sample was used to represent the encapsulation efficiency (wt %).
[0116] Preparation of contrast agent
[0117] The mixture of 86mg dipalmitoylphosphatidylcholine (DPPC), 38.4mg cholesterol and 19.2mg PEG-phospholipids (SUNBRIGHT DSPE-020CN, the lipid modified by polyethylene glycol, by NIPPON OIL & FATS CO., LTD. production) into a stainless steel autoclave, heated to keep the temperature of the still at 60°C, and then, 13g of liquid carbon dioxide was added thereto. While stirring, make the pressure in the kettle by reducing the volume inside the autoclave by 50kg...
Embodiment 2
[0124] contrast agent
[0125] The preparation of samples 2-1 to 2-8 is the same as that of sample 1-1 in Example 1, except that the amount or type of contrast medium solution is different.
[0126] The iodine content of the sample thus prepared was measured, and the encapsulation efficiency (ie, the ratio of iopamidol encapsulated inside the liposome to the total amount of iodine compound) was determined by a spectrophotometer, as shown in Table 2.
[0127] Table 2
[0128] sample
Embodiment 3
[0131] Liposome vesicle size was determined by the following method. After the dispersion containing liposome vesicles encapsulated with iodine compound was frozen and fractured, carbon was evaporated and deposited on the fracture interface, and the deposited carbon was observed by electron microscope (freeze fracture TEM method).
[0132] Particle size was defined as the arithmetic mean of the sizes of the 20 liposomal particles observed.
[0133] Preparation of contrast agent
[0134] A mixture of 192 mg of dipalmitoylphosphatidylcholine (DPPC), 76.8 mg of cholesterol and 38.4 mg of PEG-phospholipid (SUNBRIGHT DSPE-020CN, polyethylene glycol-modified phospholipid, produced by NIPPON OIL & FATS CO., LTD. ) into a stainless steel autoclave, heated to keep the temperature of the still at 60°C, and then, 13g of liquid carbon dioxide was added thereto. While stirring, make the pressure in the kettle by reducing the volume inside the autoclave by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com