Vasculostatic agents and methods of use thereof
a vasculostatic agent and vascular technology, applied in the field of vascular dysfunction treatment, can solve the problems of loss of normal function, serious pathologic consequences of compromised vasculostasis, and adverse effects on tissue and organ function and ultimately survival, so as to reduce vascular leakage, inhibit or reduce vascular leakage, and reduce vascular leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Syntheses of Vasculostatic Agents
Experimentals
General Analytical Methods
[0351] All solvents are used without further purification. Reactions are usually run without an inert gas atmosphere unless specified otherwise. All 1H NMR are run on a 500 MHz Bruker NMR. Chemical shifts are reported in delta (δ) units, parts per million (ppm) downfield from tetramethylsilane. Coupling constants are reported in hertz (Hz). A Water LC / MS system is used in identity and purity analysis. This system includes a 2795 separation module, a 996 photodidode array detector and a ZQ2000 mass spectrometer. A Zorbax SB column (150×4.6mm 3.5μ, Agilent Technologies) is used for the LC. Column temperature is 40° C. Compounds are separated using gradient elution with mobile phases of water (0.05%TFA (A)) and acetonitrile (0.05%TFA (B)). Flow rate is 1 mL / min. The gradient program used in separation is 0-15 min: 5-60% B; 15-15.5 min: 60-100% B; 15.5-17 min: 100% B.
[0352] The following gradient programs were...
example 2
Anti-Cancer Therapy with Vasculostatic Agents
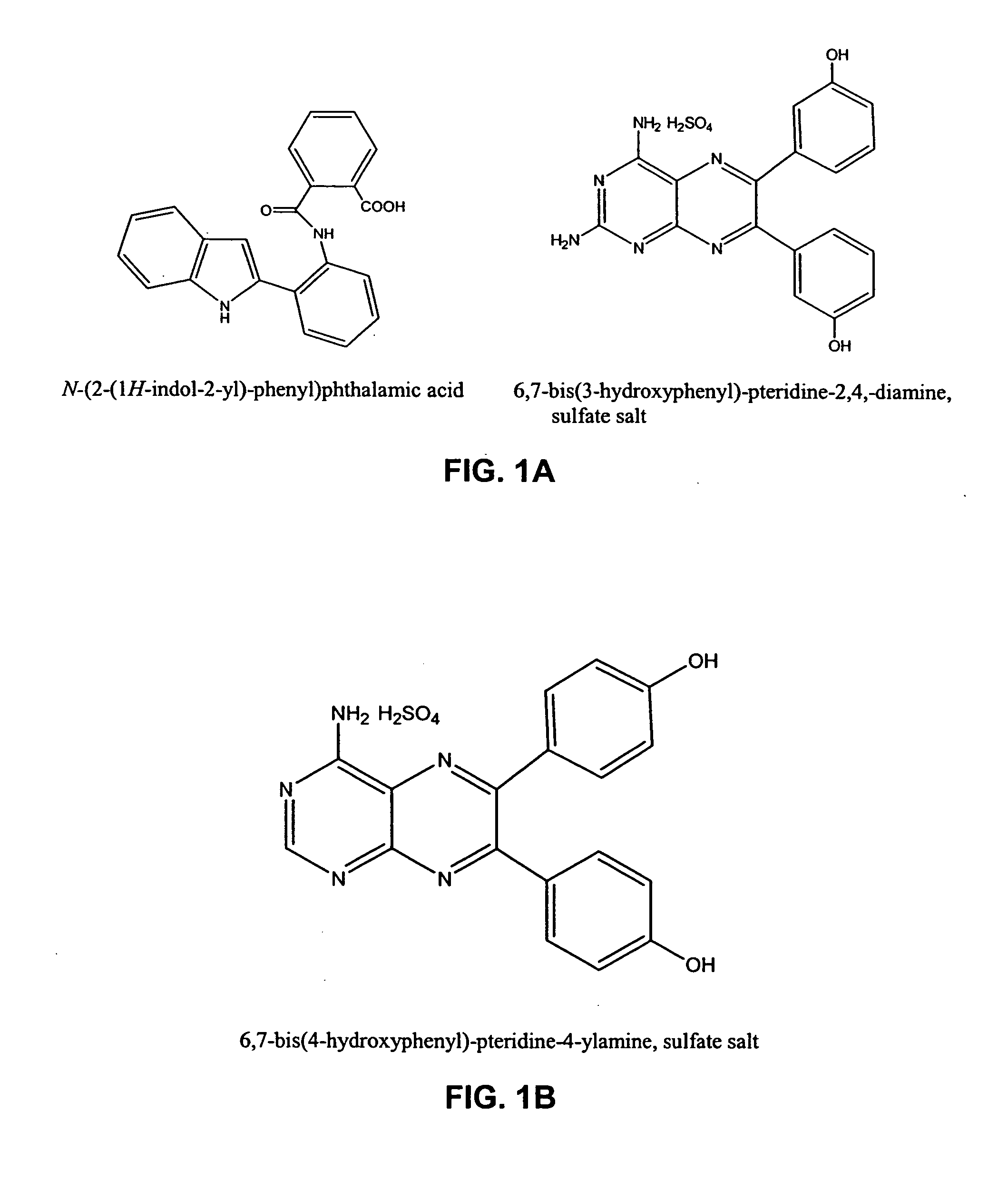
[0640] The following experiments show the use of vasculostatic agents of the invention alone and in combination with chemotherapeutic agents for treatment of cancer. FIG. 2 shows the synergistic results of co-drug therapy utilitizing 6,7-bis(4-hydroxyphenyl)-pteridin-4-ylamine, sulfate salt, (compound A—in this example formulated in 50% PEG400:50% water) illustrated in FIG. 1, with doxorubicin (in this example formulated in 50% PEG400:50% water). In the experiment shown in FIG. 2, syngeneic Lewis lung carcinoma cells were injected I.V. in order to establish lung metastases in Balb / C mice. Beginning 10 days after cells were injected, doxorubicin (3 mg / kg) and / or 6,7-bis(4-hydroxyphenyl)-pteridin-4-ylamine, sulfate salt, (compound A—various doses as shown) was given I.P. every 3 days for 3 cycles. Animals were sacrificed at day 20, lungs were collected, and weighed. Net tumor burden is the weight of tumor-bearing lungs minus the average we...
example 3
Inhibition of Vascular Permeability
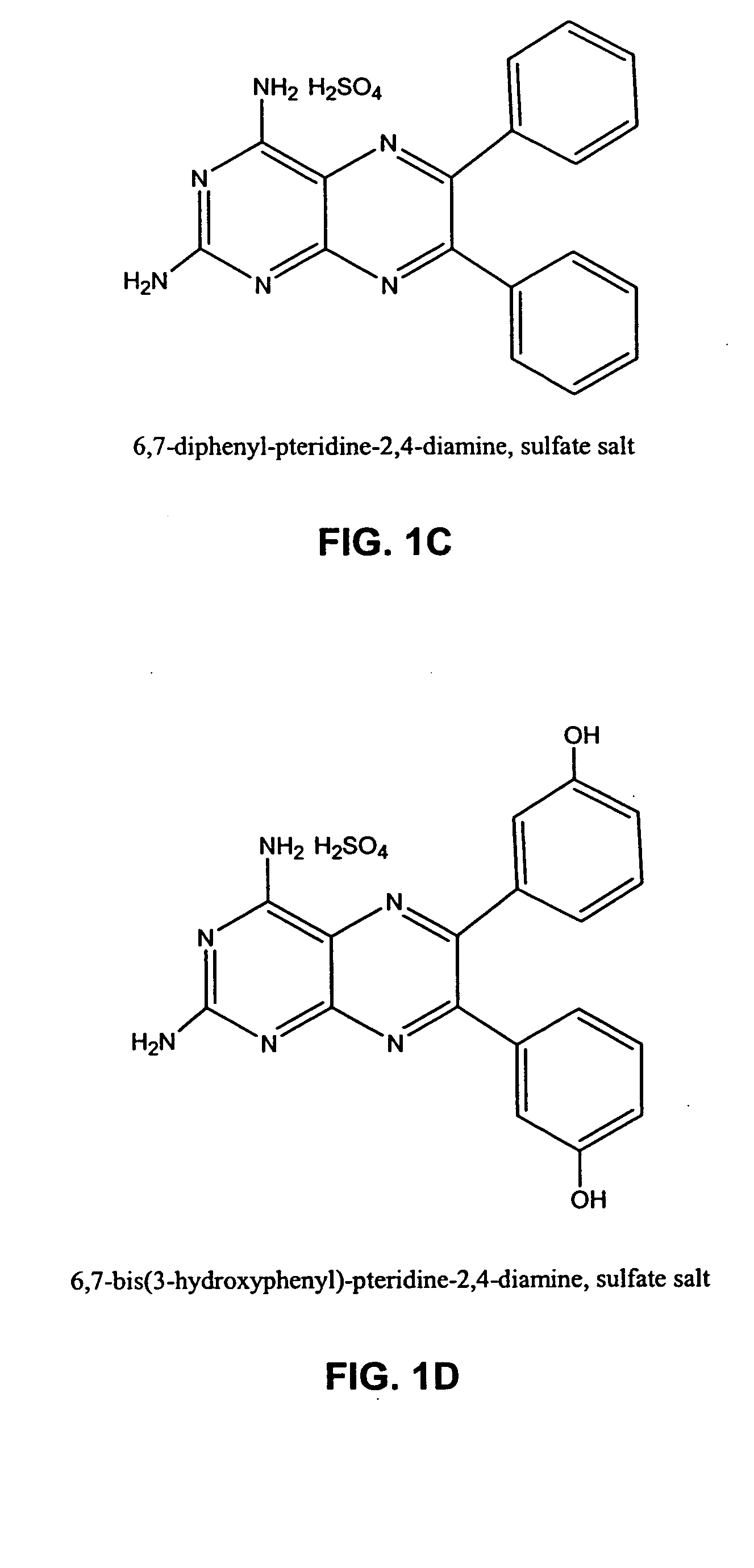
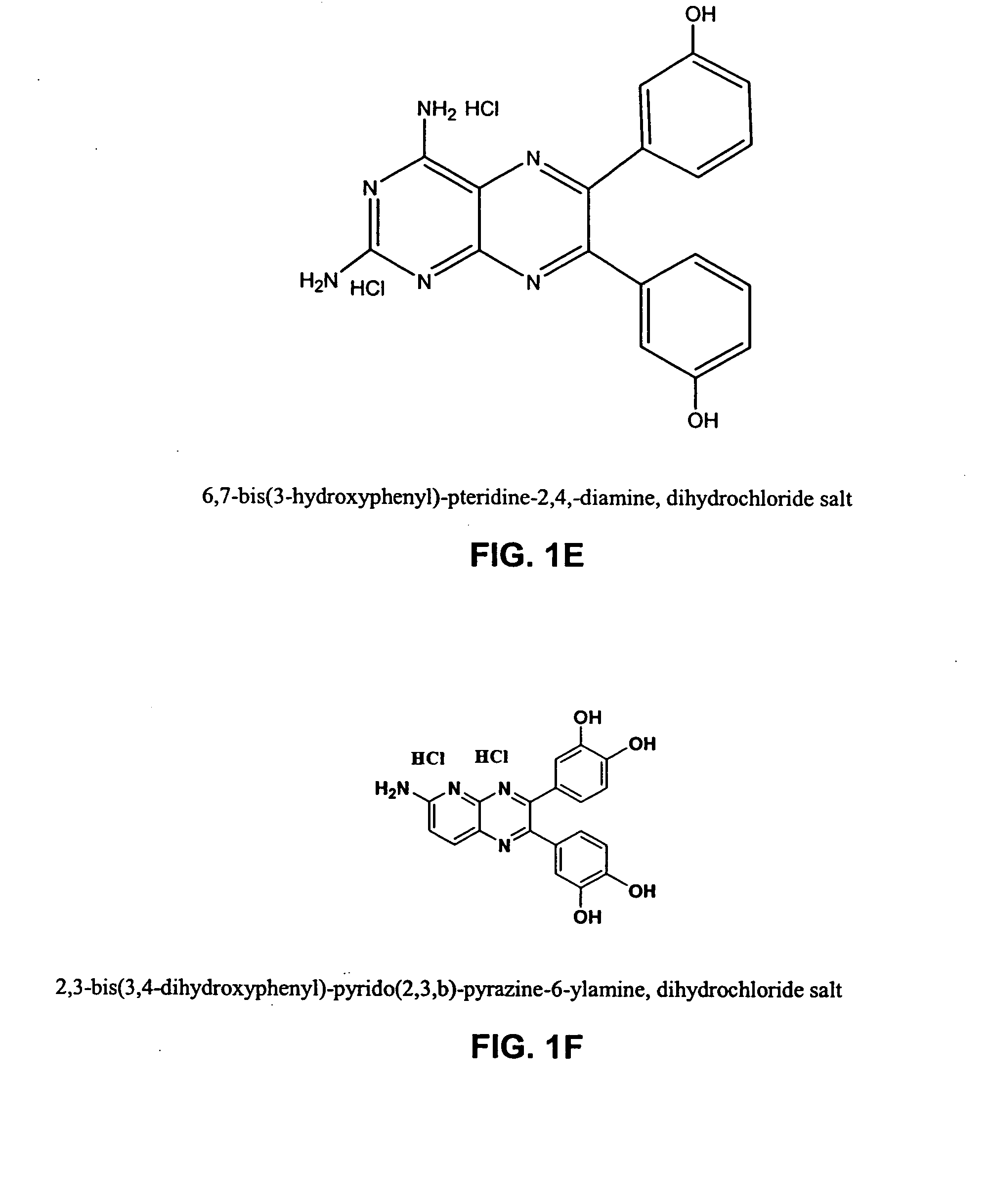
[0646] IL-2 is used clinically to treat metastatic melanoma and renal cell carcinoma and the dose-limiting toxicity for IL-2 is Vascular Leak Syndrome (VLS). Two representative examples from distinct chemotype series were selected for initial study in the reduction of IL-2-induced VLS (see FIG. 1 compounds). The compounds were pre-screened for in vivo reduction of vascular permeability and there was no observable gross toxicity as single agents at 20-fold higher doses.
[0647] The results of the studies shown in FIGS. 7-8 indicate that representative compounds of the invention show inhibition of vascular leak in vivo. There were no effects on T cell proliferation in prescribed dose range (see FIGS. 10-11) and no effects on anti-tumor activity of IL-2 (melanoma model; see FIG. 9). The following experiments exemplify the results for co-drug therapy.
[0648] BalbC mice were given 9 injections of the indicated dose of murine IL-2 (in this example formul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com