Anti-trkb monoclonal antibodies and uses thereof
a monoclonal antibody and anti-trkb technology, applied in the field of anti-trkb monoclonal antibodies, can solve the problems of no successful examples of reagents that act as potent and selective in vivo agonists of trkb, and the outcome of bdnf protein therapy in clinics has been negativ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
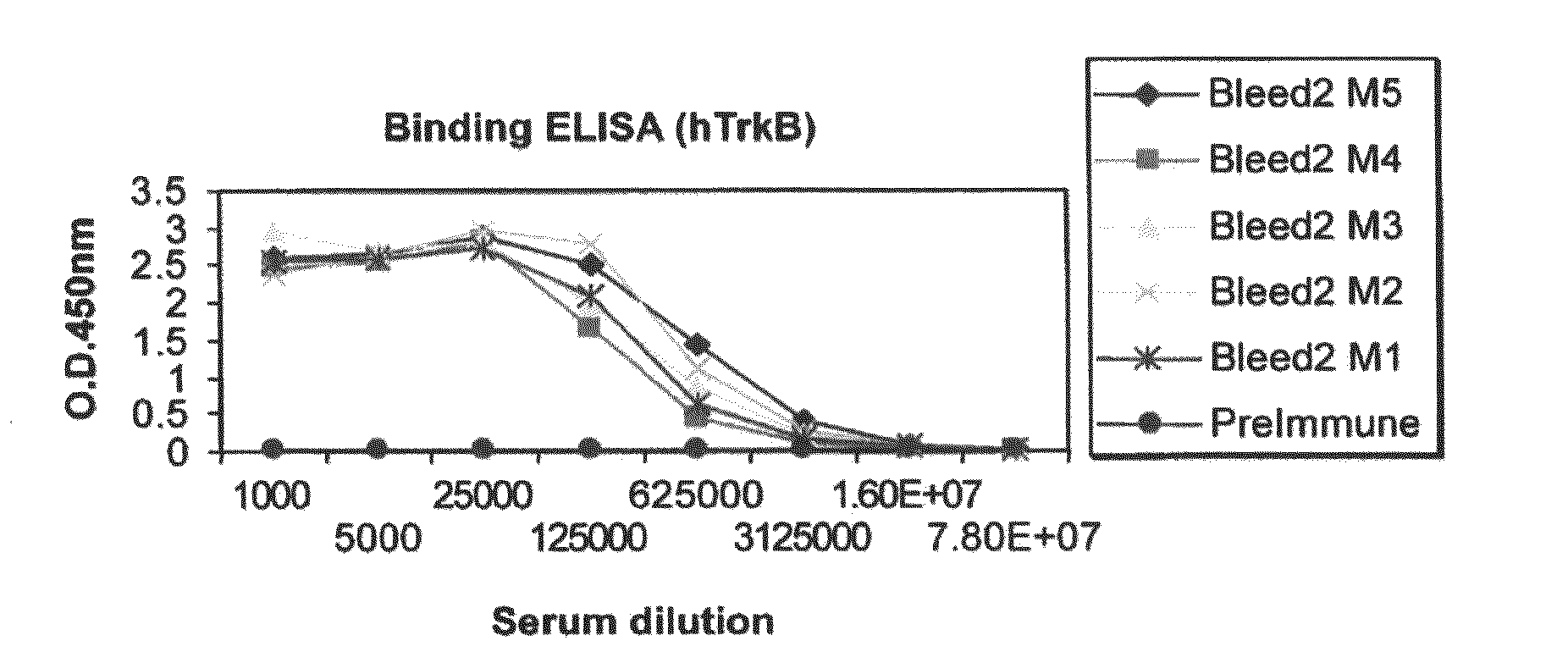
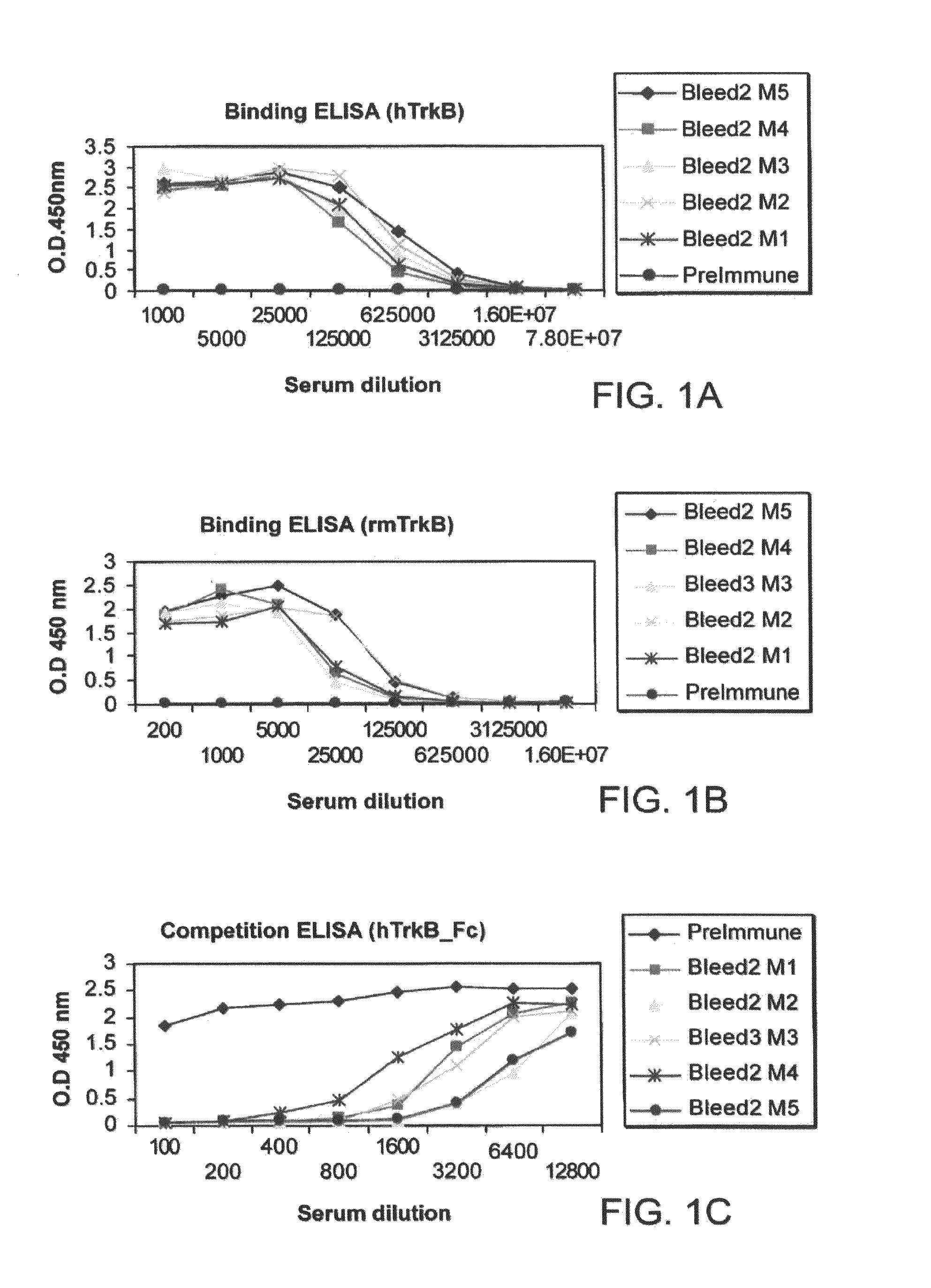
[0101]This example describes the preparation and in vitro characterization and testing of a plurality of TrkB antibodies.
Materials and Methods
Immunogens
[0102]Murine anti-TrkB antibodies were prepared using a mixture of two protein immunogens: a first recombinant protein that includes the extracellular domain (ECD) of human TrkB (rhTrkB-ECD) (R&D systems, Inc., Cat. No. 397-TR / CF) and a second recombinant protein that includes the extracellular domain of murine TrkB (rmTrkB-ECD) (R&D system, Inc., Cat. No. 1494-TB / CF).
[0103]The extracellular domain of human TrkB is comprised of amino acid residues C32-H430 of the full length protein (which is set forth as SEQ ID NO:1, GenBank Accession No. NP—006171). rhTrkB-ECD was expressed in murine myeloma cell line NSO. The calculated molecular mass of monomeric rhTrkB-ECD is 44 kDa; however, when glycosylated it migrates as a broad band of 80-100 kDa in SDS-PAGE under reducing conditions.
[0104]The extracellular domain of murine TrkB is comprise...
example 2
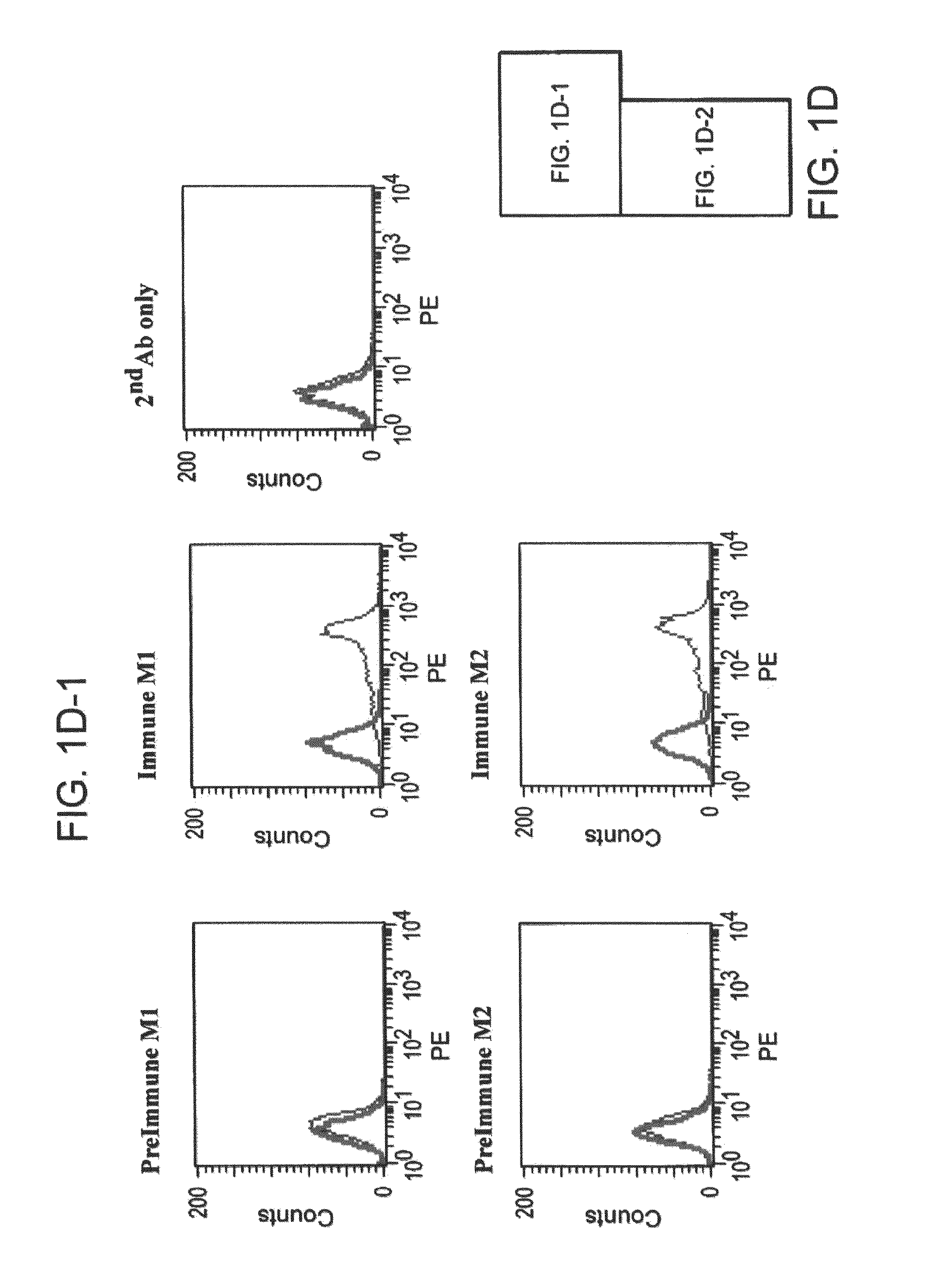
[0135]This example describes further in vitro characterization and testing of a plurality of TrkB antibodies. In particular, this example describes experiments that were performed to assess the TrkB vs. TrkA and TrkC specificity of some of the antibodies of Example 1.
Materials and Methods
FACS Analysis
[0136]HEK-293 cells expressing human TrkA were detached from the plates with PBS containing 5 mM EDTA and transferred in to 5 ml Falcon tubes (Becton Dickinson, Cat. No. 352063) with 2×105 cells per tube. Cells were washed once with PBS by centrifuging at 800 rpm at 4° C. for 3 min, and incubated for 30 min at 4° C. with 100 μl of hybridoma culture supernatant or immune serum diluted in PBS with 1% FBS. The cells were washed 3 times with 1 ml PBS containing 1% FBS and incubated for 30 min at 4° C. in the dark with PE labeled goat anti-murine IgG, F(ab′)2 fragment (DAKO Corporation, Cat. No. R0480) in PBS containing 1% FBS. Cells were washed three times again and re-suspended in 250 μl P...
example 3
[0140]This example describes the in vivo testing of a plurality of TrkB antibodies in a rodent model of neonatal hypoxia-ischemia (HI).
Materials and Methods
Animals and Surgical Procedures
[0141]The rodent model of neonatal hypoxia-ischemia (HI) was based on the Levine procedure that is set forth in FIG. 11 (e.g., see Levine, Am. J. Pathol. 36:1-17, 1960; Rice et al., Ann. Neurol., 9:131-141, 1981 and Gidday et al., Neurosci. Lett. 168:221-224, 1994 each of which is incorporated herein by reference). Briefly, pups at P7 were anesthetized with 2.5% halothane and the left common carotid artery was permanently ligated. After the incisions were sutured, pups were returned to the home cage for recovery and feeding. Two hours later, pups were placed in individual containers, through which humidified 8% oxygen was flowed. After 2.5 hrs of a hypoxic ischemic period, the pups were returned to their home cages. For treatment, animals received a 5 μl intracerebroventricular injection of 0.1 or 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com