Bis-Choline Tetrathiomolybdate for Treating Wilson Disease
a technology of wilson disease and tetrathiomolybdate, which is applied in the direction of heavy metal active ingredients, drug compositions, metabolic disorders, etc., can solve the problems of impaired biliary excretion of cu, impaired incorporation, and organ damage and dysfunction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Bis-choline Tetrathiomolybdate in Patients with Wilson'S Disease: an Open-Label, Multicentre, Phase 2 Study
Background
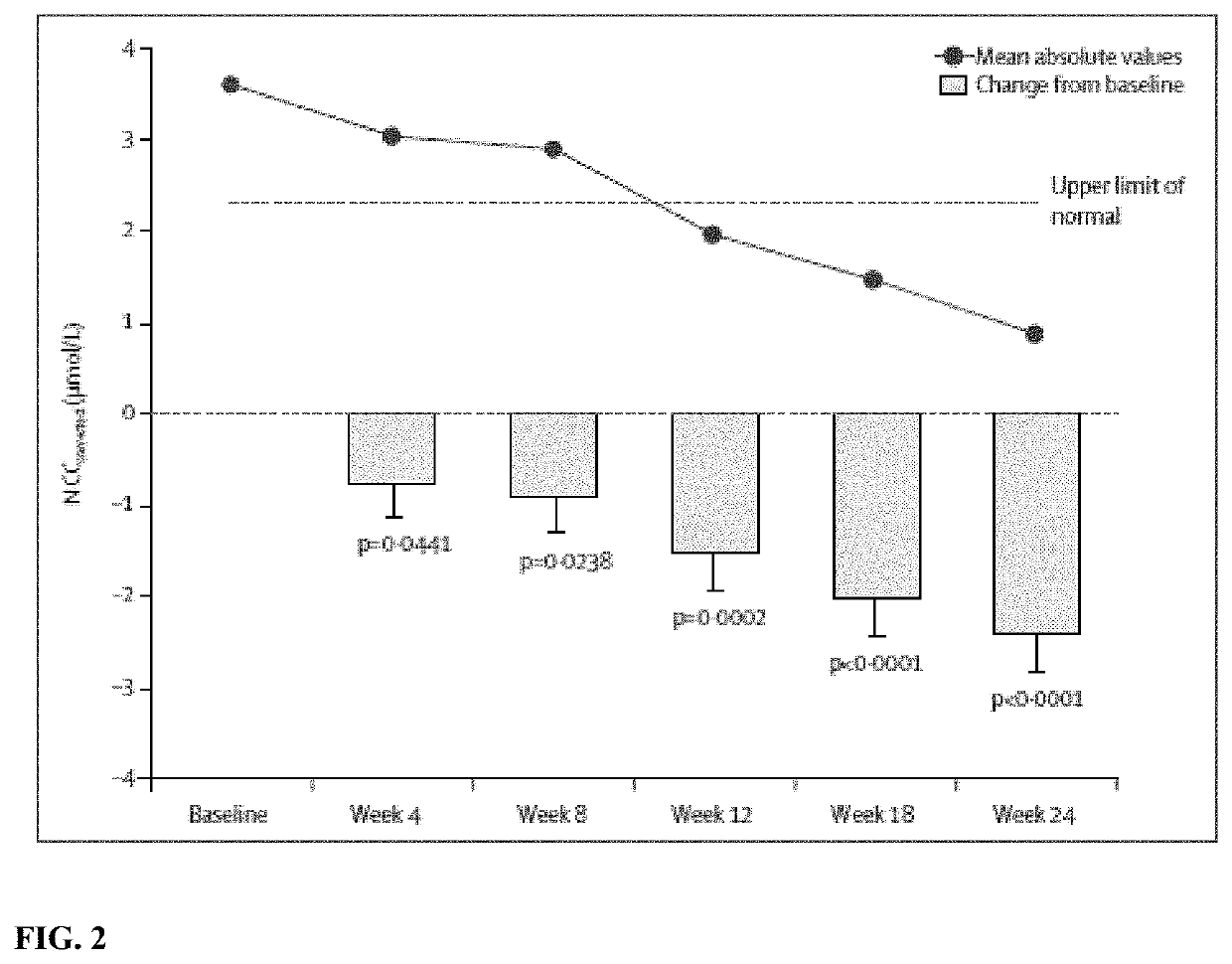
[0153]Wilson's disease is a genetic disorder in which copper accumulates in the liver, brain, and other tissues. Therapies had been limited by efficacy, safety concerns, and multiple daily dosing. Bis-choline tetrathiomolybdate (WTX101) is an oral, first-in-class, copper-protein-aggregating molecule that targets hepatic intracellular copper and reduces plasma non- ceruloplasmin-bound copper (NCC) by forming tripartite complexes with albumin and by increasing biliary copper excretion. The efficacy and safety of WTX101 was assessed in the initial or early treatment of patients with Wilson's disease.
Methods
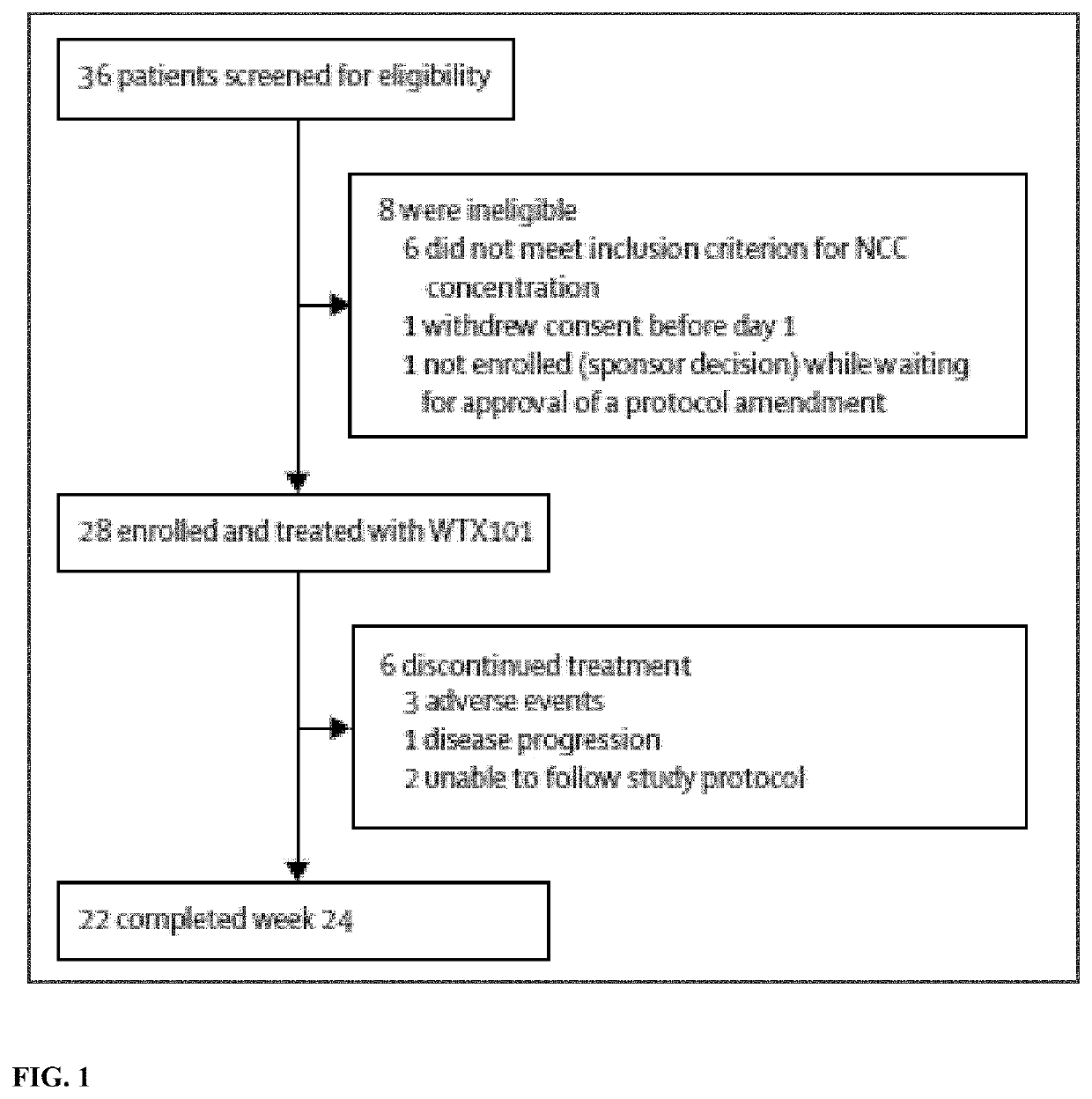
[0154]This open-label, phase 2 study was performed at 11 hospitals in the USA and in Europe. Patients (≥18 years) with Wilson's disease were enrolled who were untreated or who had received no more than 24 months of treatment with chelators or zinc, had a Leipzig score ...
example 2
Long-Term Efficacy and Safety of WTX101 in Wilson Disease: Data from an Ongoing Extension of a Phase 2 Study
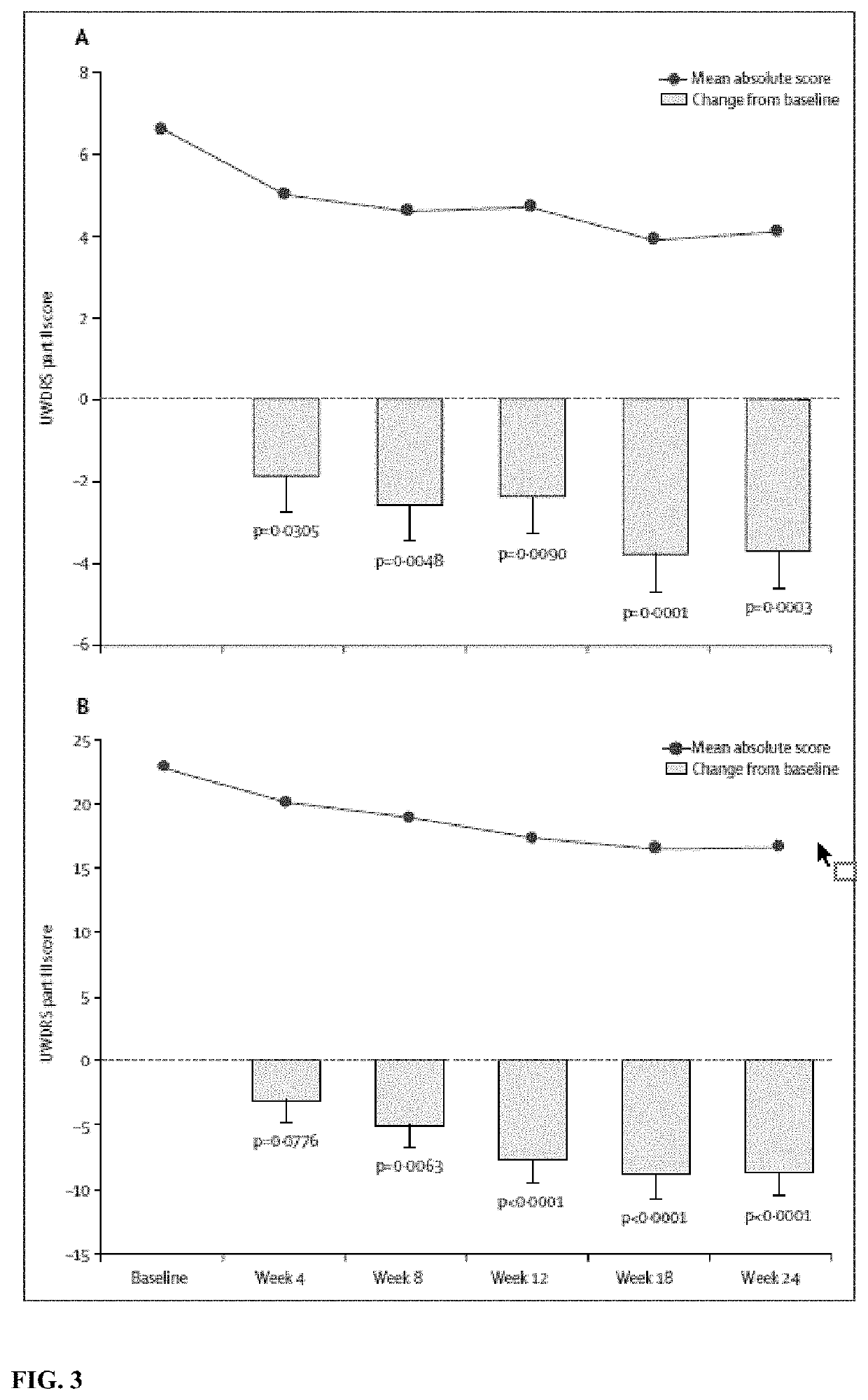
[0191]In Example 1, oral once-daily WTX101 monotherapy rapidly lowered and controlled NCC, improved disability and neurological status, without early drug-induced neurological worsening and stabilized liver function in patients with WD after 24 weeks. 72-week efficacy and safety data from the ongoing extension period of the phase 2 study represents the first prospective report on long-term disease control with WTX101 in WD.
[0192]Example 1 was an open-label multicenter single-arm phase 2 trial conducted in 28 adults with a diagnosis of WD established by a Leipzig score of ≥4. For inclusion, NCC levels had to be above the lower limit of the normal reference range (≥0.8 μM). Participants had either no prior treatment for WD (n=9) or ≤24 months' prior treatment with chelation or zinc (<28 days, n=9; 28 days to 2 years, n=10). Participants received WTX101 for 24 weeks using a respo...
example 3
A Phase 3, Randomised, Rater-Blinded, Multi-Centre Study to Evaluate the Efficacy and Safety of WTX101 Administered for 48 Weeks Versus Standard of Care in Wilson Disease Subjects Aged 18 and Older with an Extension Phase of up to 60 Months
Summary of Study Design
[0203]A randomised, rater-blinded, multi-centre study assessing the efficacy and safety of an individualised WTX101 dosing regimen administered for 48 weeks, compared to SoC, will be performed in WD subjects aged 18 and older.
[0204]Approximately 102 subjects will be enrolled at approximately 5 to 10 North American sites and 15 to 25 sites in the Rest of the World.
[0205]Eligible subjects with WD, who have received SoC therapy (i.e., chelation therapy with penicillamine or trientine, Zn therapy, or a combination of both chelation and Zn therapy) for >28 days (Cohort 1), or who are treatment naive or who have received SoC therapy (i.e., chelation therapy with penicillamine or trientine, Zn therapy, or a combination of both chel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| threshold | aaaaa | aaaaa |
| threshold | aaaaa | aaaaa |
| threshold | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap