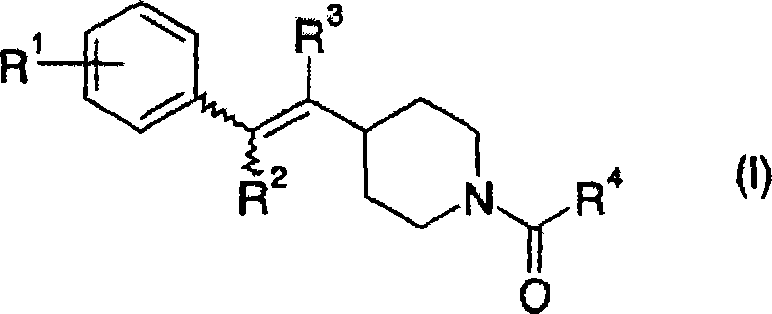
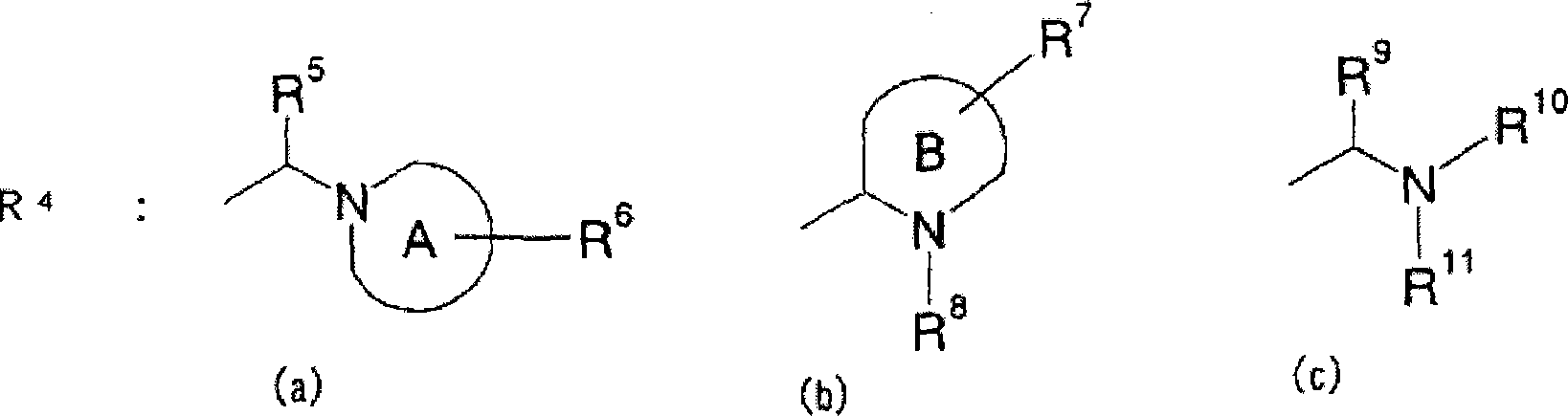
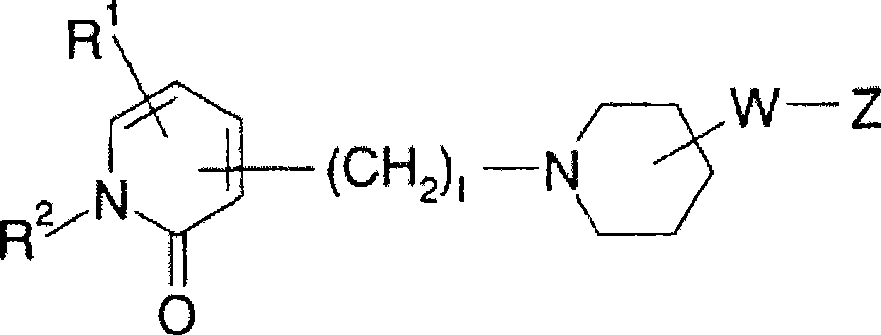Piperidine derivative or pharmaceutically acceptable salt thereof
A derivative and pharmaceutical technology, applied in the field of pharmaceutical compositions, can solve the problem of not mentioning or suggesting sodium channel inhibitory effect or analgesic effect, etc., and achieve excellent sodium channel inhibitory effect, high analgesic effect, and excellent analgesic effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] To 15.0 ml of a N,N-dimethylformamide suspension containing 2.38 g of (2-chlorobenzyl)(triphenyl)phosphonium chloride, 0.63 g of potassium tert-butoxide was added. The mixture was stirred at room temperature for 10 minutes. To the obtained orange suspension, 1.00 g of tert-butyl 4-formylpiperidine-1-carboxylate was added, and the reactant was stirred for 15 minutes to carry out the reaction. The resulting reaction mixture was poured into a saturated aqueous solution of ammonium chloride, and extracted with ethyl acetate. The organic layer was washed with brine, then dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (n-hexane-ethyl acetate) to give 1.45 g of tert-butyl 4-[2-(2-chlorophenyl)vinyl]piperidine-1-carboxylate in the form of a colorless oil .
Embodiment 2-32
[0092] Compounds shown in Tables 1-3 were obtained in the same manner as in Reference Example 1.
Embodiment 33
[0094] To 1.45 g of tert-butyl 4-[2-(2-chlorophenyl)vinyl]piperidine-1-carboxylate was added 2.10 g of p-toluenesulfonic acid. The mixture was stirred at 150° C. for 5 hours to conduct a reaction. 10.0 ml of water was added to the resulting reaction mixture. The mixture was adjusted to pH about 11 with 10% aqueous sodium hydroxide solution, and extracted with chloroform. The organic layer was washed with water and brine, then dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain 0.97 g of 4-[(E)-2-(2-chlorophenyl)vinyl]piperidine in the form of a yellow oil.
[0095] (Reference Examples 34-40)
[0096] Compounds shown in Table 3 were obtained in the same manner as in Reference Example 33.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap