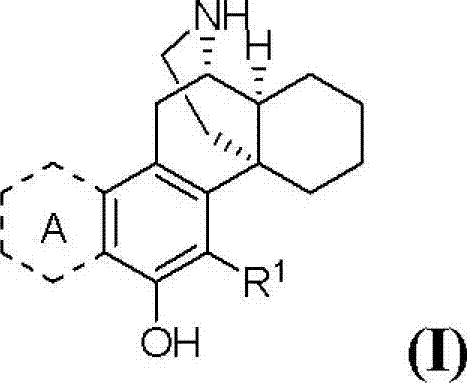
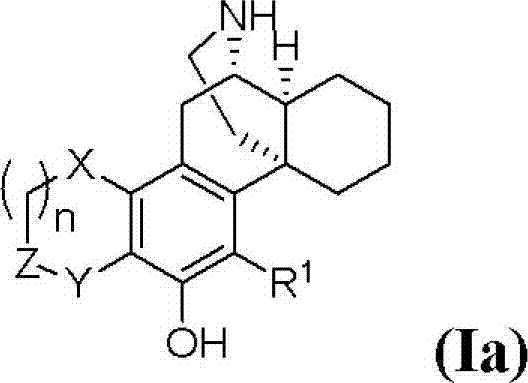
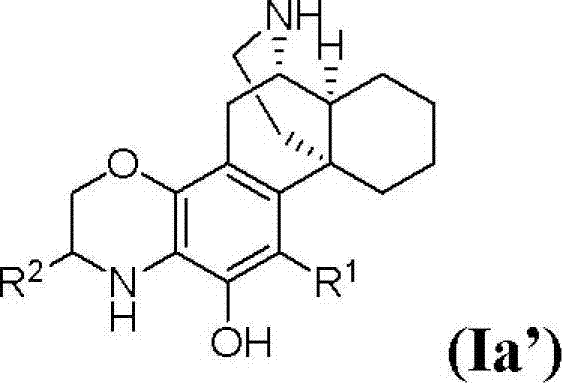
(+)-3-hydroxymorphinan-based polycycle derivatives as neuroprotectants
A hydroxyl, neurodegenerative technology, applied in nervous system diseases, drug combinations, medical preparations containing active ingredients, etc., can solve problems such as lack of efficacy or safety of drugs, and failure to achieve clinical efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] (6S, 6aS, 10aS)-2,3,4,5,6,6a,7,8,9,10-decahydro-6,10a-(cycloimine bridge ethylene)phenanthrene[2,1 -b][1,4]oxazin-12-ol TFA salt (11)
[0161] step 1:
[0162] (4bS, 8aS, 9S)-benzyl 3-hydroxy-6,7,8,8a,9,10-hexahydro-5H-9,4b-(cycloiminoethylene)phenanthrene-11-carboxylic acid Esters (2)
[0163]
[0164] To (4bS, 8aS, 9S)-6,7,8,8a,9,10-hexahydro-5H-9,4b-(cycloimine bridge ethylene)phenanthrene-3-alcohol HBr(1)(50.0 g, 154 mmol) and sodium hydroxide (12.3 g, 308 mmol) in 1,4-dioxane (500 mL) and water (500 mL) was added Cbz-Cl (24.2 mL, 170 mmol) dropwise. The reaction mixture was vigorously stirred overnight at room temperature. After the reaction was complete, water (200 mL) was added. The mixture was extracted with ether (500 mL x 2). The combined organic phases were washed with MgSO 4 Dry, filter and evaporate under vacuum. The residue was subjected to flash column chromatography (Biotage SP1 TM ) to afford the title compound (54.6 g, 94%) as a white solid...
Embodiment 2
[0213] (7S, 7aS, 11aS)-3, 4, 5, 6, 7, 7a, 8, 9, 10, 11-decahydro-2H-7, 11a- (cycloimine bridge ethylene) phenanthrene [2 , 1-b][1,4]oxazepine -13-alcohol TFA salt (12)
[0214]
[0215] Using N-(3-bromopropyl)phthalimide (13) to replace N-(2-bromoethyl)phthalimide (7), repeat the steps of Example 1 to prepare Example 2 compounds.
[0216] 1 H NMR (400MHz, CD 3 OD) 4.14-4.13(m, 1H), 4.07-4.06(m, 1H), 3.74-3.72(m, 1H), 3.35-3.33(m, 2H), 3.06(dd, J=12.8, 2.8Hz, 1H), 2.97-2.90(m, 1H), 2.75(td, J=13.2, 3.6Hz, 1H), 2.68-2.63(m, 1H), 2.36-2.33(m, 1H), 2.10-1.99(m, 3H), 1.85(dt, J=12.4, 2.8Hz, 1H), 1.78-1.65(m, 2H), 1.58-1.45(m, 4H), 1.42-1.21(m, 4H), 1.16-1.06(m, 1H).
[0217] MH+315.
Embodiment 3
[0219] 1-((6S, 6aS, 10aS)-12-hydroxyl-2,3,6,6a,7,8,9,10-octahydro-6,10a-(cycloimine bridge ethylene)phenanthrene[ 2,1-b][1,4]oxazin-4(5H)-yl)ethanone TFA salt (15)
[0220] step 1:
[0221] (6S, 6aS, 10aS)-benzyl 4-acetyl-12-(benzyloxy)-2,3,4,5,6,6a,7,8,9,10-decahydro-6,10a- (Cycloiminoethylene)phenanthrene[2,1-b][1,4]oxazine-15-carboxylate (14)
[0222]
[0223] (6S, 6aS, 10aS)-benzyl 12-(benzyloxy)-2,3,4,5,6,6a,7,8,9,10-decahydro-6,10a- (Cycloiminoethylene)phenanthrene[2,1-b][1,4]oxazine-15-carboxylate (10) (120mg, 0.229mmol), DMAP (33.6mg, 0.275mmol) and To a solution of DIPEA (0.36 mL, 2.06 mmol) in DCM (20 mL) was added acetyl chloride (0.1 mL, 1.37 mmol). The reaction mixture was stirred overnight at 50 °C. The reaction mixture was evaporated under vacuum to remove solvent. The residue was subjected to flash column chromatography (Biotage SP1 TM ) to afford the title compound (96 mg, 74%) as a brown gum.
[0224] MH+567.
[0225] Step 2: 1-((6S,6aS,10S)-12-Hydr...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap