Matrix metal proteinase-2 polypeptide inhibitors and application thereof
A peptide inhibitor and matrix metal technology, applied in the field of matrix metalloproteinase-2 peptides, can solve the problems of side effects, lack of specificity of small molecule inhibitors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
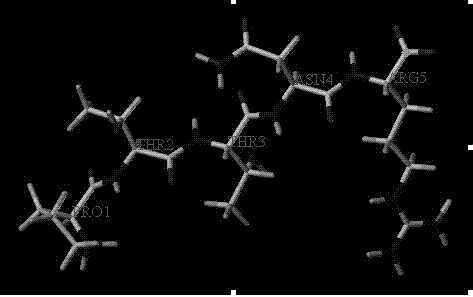
[0017] Polypeptide chemical synthesis method
[0018] Polypeptides are synthesized by Fmoc chemical method. The synthesis reaction proceeds from the C-terminus to the N-terminus. The Rink medium (available from Advanced ChemTech) has free amino groups, and Arg, Asn, Thr, Thr and Pro are sequentially connected. In each step of the connection process, the amino acid residues must be activated. The activation mixture contains 4 times the free amino groups on the medium of HBTU, HOBt, DIEA and Fmoc-amino acids. After each amino acid ligation reaction, a mixture of pyridine / acetic acid / N-methylimidazole (4:1:0.5) was used to block the unlinked free amino groups, and the blocking reaction was 10 minutes. After each amino acid ligation reaction, before the next amino acid is ligated, the Fmoc- group on the medium must be removed. To remove the Fmoc- group, use 20% piperidine-containing dimethylformamide, which takes 15 minutes. Finally, when all the amino acid residues are connected s...
Embodiment 2
[0022] Peptide inhibitors have IC50 values for several target enzymes in vitro: matrix metalloproteinase-3, -8, -2 and tumor necrosis factor releasing enzyme (both purchased from sigma).
[0023] Recombinant human matrix metalloproteinase-2 is expressed in E. coli cells. The enzyme is activated with 0.01 μM matrix metalloproteinase-3 active site. The buffer used is 100 mM Tris / HCl, pH 7.4, 100 mM NaCl, 10 mM CaCl 2 and 0.01% Tween-20. The concentration of MMP-2 during activation is 72 ng / μl (1 μM). The enzyme activity is detected by cutting the fluorescence to produce the peptide substrate Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH 2 And detection of the generated fluorescence value is achieved (excitation wavelength = 328 nm, detection wavelength = 392 nm). All detections are performed in a 100 μl reaction system at 37°C. The measured IC50 value is 54.97 μmol.
[0024] The detection of recombinant human matrix metalloproteinase-8 is similar to that of -2 and uses the same fluorescen...
Embodiment 3
[0028] Using endotoxin shock model to detect the in vivo activity of peptide inhibitors
[0029] Before establishing the endotoxin shock model, we first determined the LD50 of LPS (E. coli 0111: B4) mice as 50 μg per mouse. In the experiment, we used 100 μg per mouse. In this way, the control group All of the mice can die. A positive control experiment was done with Regasepin2. The mice in the control group were injected with 100 μg LPS, and the mice in the Regasepin2 experimental group were injected with 0.7 mg of peptide 5 minutes after the LPS injection. By creating a Kaplan-Meier survival curve, it was found that Regasepin2 can effectively protect mice injected with 100 μg and improve the survival rate. After successfully establishing an endotoxin shock model in mice, the model was used to test the in vivo viability of Pro-Thr-Thr-Asn-Arg. The mice in the control group (12 mice) were injected with 100 μg LPS, while the mice in the peptide inhibitor group (12 mice) were inj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com