A kind of pharmaceutical composition for treating cerebrovascular disease
A technology for cerebrovascular diseases and compositions, applied in the field of medicine, can solve problems such as limited value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
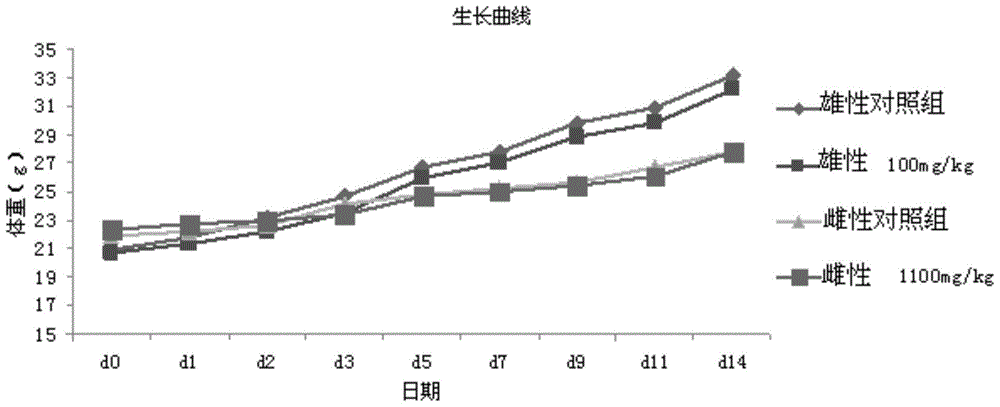
[0031] Example 1. The effect of the combination of tristilbene on cerebral ischemia-reperfusion injury in rats
[0032] 1 medicine
[0033] 1.1 Test drug preparation
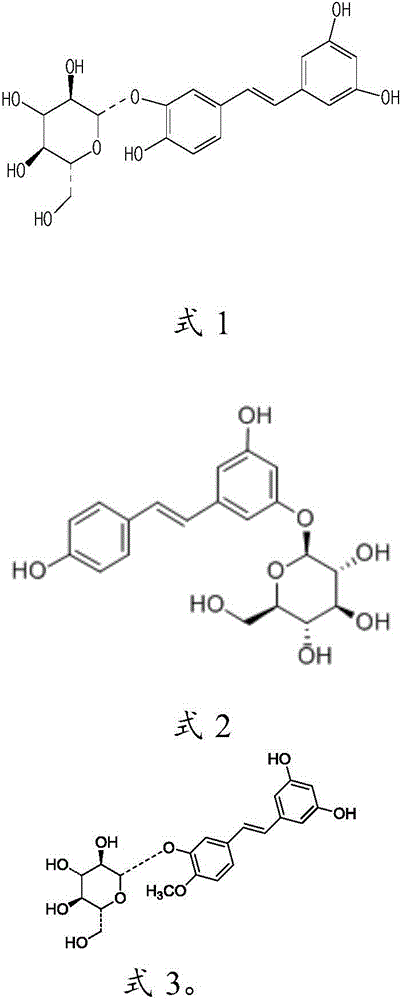
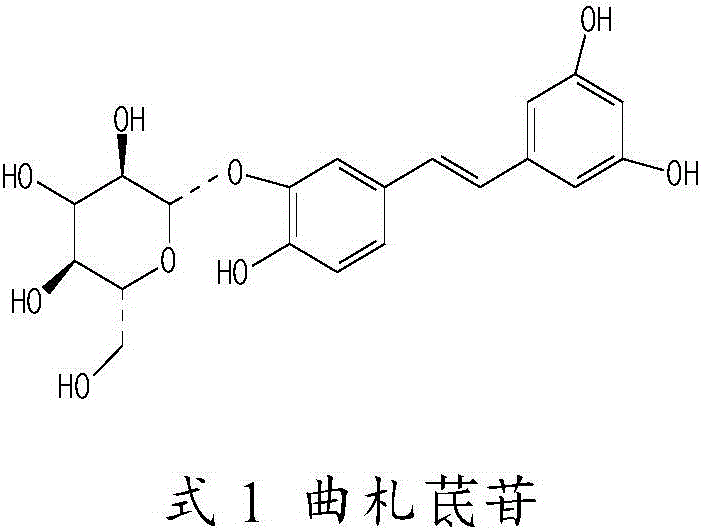
[0034] Take 6ml of water for injection respectively, heat to 80°C, add 2g of hydroxypropyl-β-cyclodextrin, stir to dissolve, add 150mg of tristilbene, polydipside, tristilbene derivatives and tristilbene respectively The composition was stirred to dissolve, 90 mg of sodium chloride was added for stirring and dissolved, and finally water for injection was added to 10 ml, and the pH value of the semi-finished product was measured (5.0-6.0) to obtain a 15 mg / ml solution, which was sequentially diluted to obtain a 3.75 mg / ml solution for use.
[0035] Positive control drug: Edaravone injection, source: Nanjing Xiansheng TECO Pharmaceutical Co., Ltd., batch number: 80-101207
[0036] Specifications: 5ml: 10mg, Properties: Colorless or almost colorless clear liquid, ready for use.
[0037] 1.2 Reagents
[0038] Re...
Embodiment 2
[0074] Example 2: Protective effect of tristilbene composition on nerve cell damage caused by hypoxia and glucose deficiency
[0075] 1 Experimental material
[0076] 1.1 Test sample
[0077] Preparation: After weighing an appropriate amount of tristilbene composition, add 0.01M PBS to the required volume to obtain a high-dose test product (1mg / ml); then filter and sterilize through a 0.2μm filter, and use PBS in a certain proportion for corresponding Dilute to obtain each dose of test substance.
[0078] 1.2 Positive Control 1
[0079] Name: Nimodipine
[0080] Source: Sigma
[0081] Lot number: SLBB1165V
[0082] Appearance: yellow powder
[0083] Preparation: Weigh an appropriate amount of sample and add DMSO to the required volume (3mg / ml); then filter and sterilize it through a 0.2 μm filter, and dilute it with PBS according to a certain proportion to obtain the required dose of the test product.
[0084] 1.3 Negative controls
[0085] Name: Sterile PBS
[0086] ...
Embodiment 3
[0123] Example 3: Mice maximum tolerated dose study of tristilbene composition
[0124] 1 Experimental material
[0125] 1.1 Test sample
[0126] Preparation: Take about 30ml of water for injection, heat it to 80°C, add 10g of hydroxypropyl-β-cyclodextrin, stir to dissolve, then add 3g of Tristilbene No. 1 composition, stir to dissolve, and then add 0.45g Sodium chloride is stirred to dissolve, and finally water for injection is added to make up to 50ml. After measuring the pH value (5.0-6.0) and content of the semi-finished product, it is finely filtered until it is clear and ready for use.
[0127] 1.2 Negative control
[0128] Name: Hydroxypropyl-β-cyclodextrin
[0129] Source: Kunming Pharmaceutical Group Co., Ltd.
[0130] Preparation: take about 30ml of water for injection, heat it to 80℃, add 10g of hydroxypropyl-β-cyclodextrin, stir to dissolve, then add 0.45g of sodium chloride and stir to dissolve, and finally add water for injection to make 50ml , After measuri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com