A pharmaceutical composition for treating cerebrovascular diseases
A technology for cerebrovascular disease and composition, applied in the field of medicine, can solve problems such as limited value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1, the effect of the combination of stilbeneside on cerebral ischemia-reperfusion injury in rats
[0031] 1 drug
[0032] 1.1 Test drug preparation
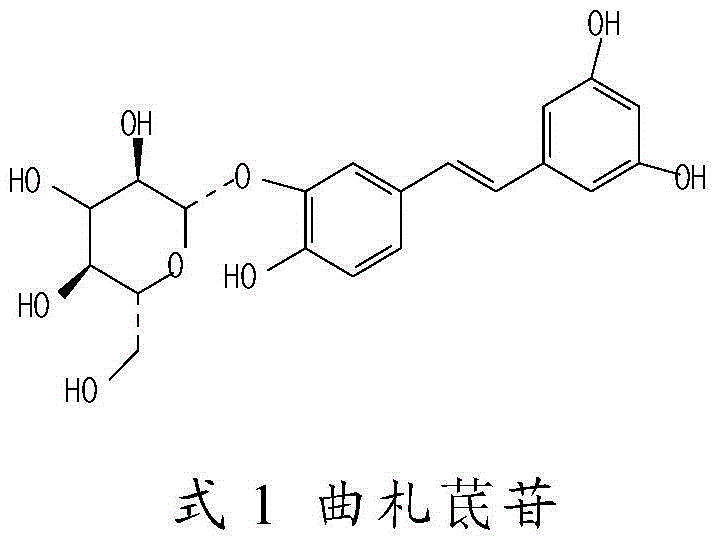
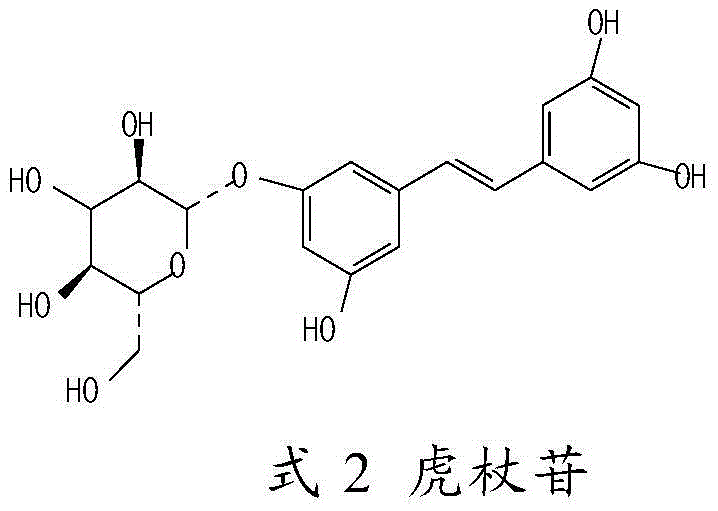
[0033] Take 6ml of water for injection, heat to 80°C, add 2g of hydroxypropyl-β-cyclodextrin, stir to dissolve, add 150mg of tristilbene, polydatin, tristilbene derivatives and tristilbene respectively Stir the composition to dissolve, add 90mg of sodium chloride and stir to dissolve, finally add water for injection to 10ml, measure the pH value of the semi-finished product (5.0-6.0), and obtain a 15mg / ml solution, and then dilute in turn to obtain a 3.75mg / ml solution for later use.
[0034] Positive control drug: Edaravone injection, source: Nanjing Xiansheng Dongyuan Pharmaceutical Co., Ltd., batch number: 80-101207
[0035] Specifications: 5ml: 10mg, traits: colorless or almost colorless clear liquid, ready for use.
[0036] 1.2 Reagents
[0037] Red tetrazolium (TTC), China Pharmaceutical Group Shanghai Ch...
Embodiment 2
[0072] Example 2: Protective effect of tristilbene glycoside composition on nerve cell damage caused by hypoxia and glucose deficiency
[0073] 1 Experimental materials
[0074] 1.1 Test sample
[0075] Preparation: After weighing an appropriate amount of tristilbene glycoside composition, add 0.01M PBS to the required volume to obtain a high-dose test product (1mg / ml); afterward, filter and sterilize through a 0.2μm filter, and use PBS in a certain proportion for corresponding Diluted to obtain each dose of the test article.
[0076] 1.2 Positive control substance 1
[0077] Name: Nimodipine
[0078] Source: Sigma
[0079] Batch number: SLBB1165V
[0080] Appearance: yellow powder
[0081] Preparation: Weigh an appropriate amount of sample and add DMSO to the required volume (3mg / ml); then filter and sterilize through a 0.2μm filter, and dilute with PBS in a certain proportion to obtain the required dose of the test product.
[0082] 1.3 Negative control substance
[...
Embodiment 3
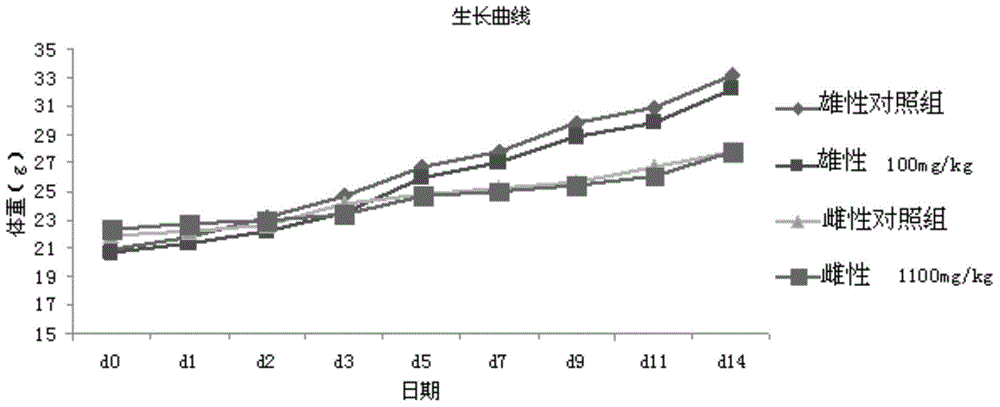
[0120] Example 3: Study on the Maximum Tolerable Dose of Trizaperoside Composition in Mice
[0121] 1 Experimental materials
[0122] 1.1 Test sample
[0123] Preparation: Take about 30ml of water for injection, heat it to 80°C, add 10g of hydroxypropyl-β-cyclodextrin, stir to dissolve, then add 3g of tristilbeside No. 1 composition, stir to dissolve, then add 0.45g Sodium chloride is stirred and dissolved, and finally water for injection is added to make the finished product 50ml. After the pH value (5.0-6.0) of the semi-finished product is measured and the content is qualified, it is finely filtered until it is clear and ready for use.
[0124] 1.2 Negative control
[0125] Name: Hydroxypropyl-β-cyclodextrin
[0126] Source: Kunming Pharmaceutical Group Co., Ltd.
[0127] Preparation: Take about 30ml of water for injection, heat it to 80°C, add 10g of hydroxypropyl-β-cyclodextrin, stir to dissolve, then add 0.45g of sodium chloride and stir to dissolve, and finally add w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com