A controllable modified amphiphilic block copolypeptide, its preparation method and its application
An amphiphilic block and copolymerization technology, applied in the field of polymer materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthesis of polyethylene glycol-b-poly(γ-propargyl-L-glutamic acid) (PEG-b-PPLG) comprises the following steps:
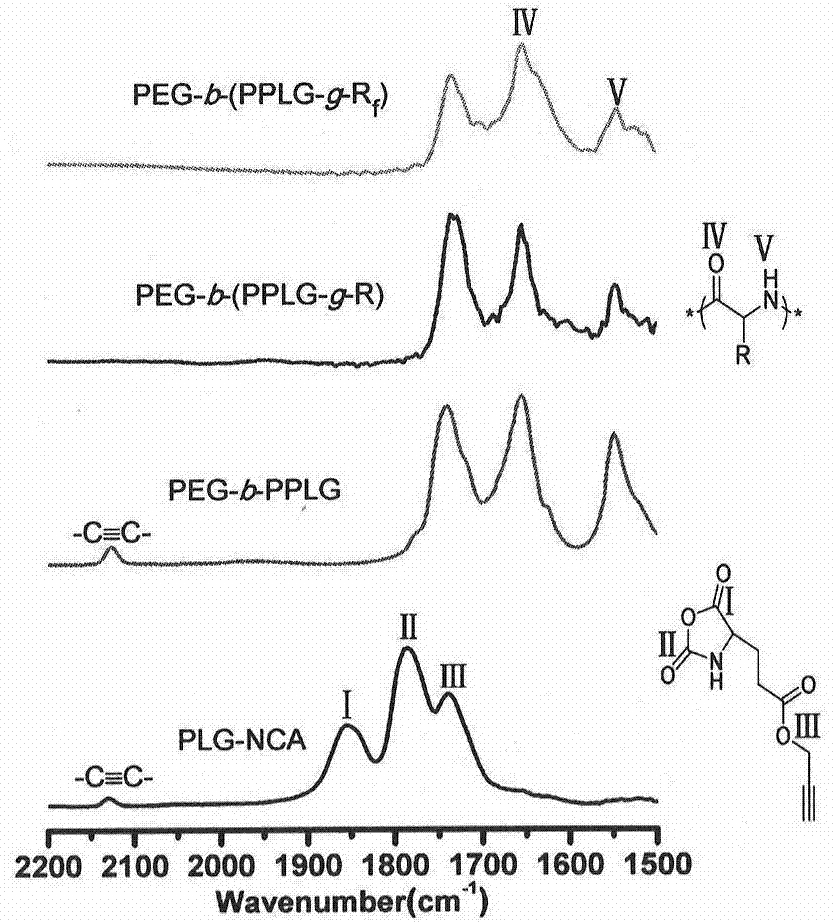
[0048] Monomethoxypolyethylene glycol primary amine (mPEG-NH 2 , 0.50g, 0.139mmol) dried in vacuum at 50°C for about six hours, and then cooled to room temperature. γ-propargyl-L-glutamic acid-N-carboxy-cyclic anhydride (PLG-NCA) (0.634 g, 3.00 mmol) was dissolved in 12.7 mL of dry DMF in the glove box, and then transferred with a syringe into the reaction system (monomer / initiator=22:1). After four days of reaction at room temperature under the protection of nitrogen, it was precipitated with ether and dried in vacuo overnight to obtain the product polyethylene glycol-b-poly(γ-propargyl-L-glutamic acid) (PEG-b-PPLG) (0.70 g, harvested rate: 85%).
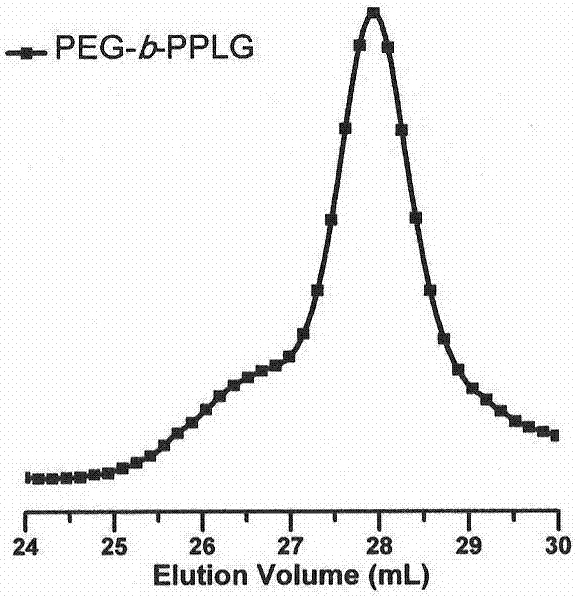
[0049] attached figure 1 The copolypeptide synthesized in Example 1 was characterized by SEC / LLS using 0.02M LiBr DMF solution as the mobile phase, and its molecular weight was about 6500g / mol, and its m...
Embodiment 2
[0051] PEG-b-PPLG is modified by mercapto-alkyne photochemical reaction, which includes the following steps:
[0052] A typical mercapto-yne chemical modification process is described as follows: Diblock copolymer PEG-b-PPLG, excess mercapto compound 1-mercaptooctane or 1-mercapto-1H, 1H, 2H, 2H-perfluorooctane, After dissolving in an appropriate amount of THF, the photoinitiator 2,2-dimethoxy-2-phenylacetophenone (DMPA) was added and reacted at room temperature for about four hours under 365 nm ultraviolet light. After the reaction, the reaction solution was precipitated in excess n-hexane, and the precipitate was further washed with n-hexane several times to remove excess unreacted raw mercapto compounds. Finally, after drying the solid product in vacuum, the two-block copolymer PEG-b-(PPLG-g-R) modified by octyl group and the two-block copolymer PEG-b-(PPLG-g-R modified by perfluorooctane) were respectively obtained. f ).
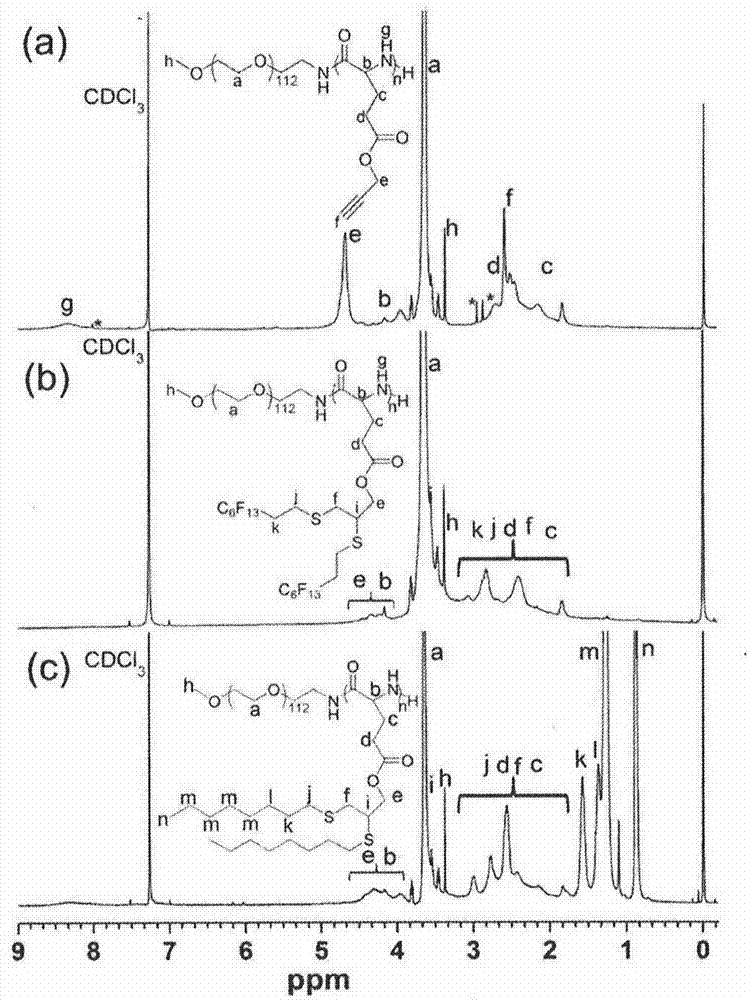
[0053] by attaching figure 2 b. figure 2 c's...
Embodiment 3
[0057] The solution self-assembly of diblock copolymer, it comprises the following steps:
[0058] The self-assembled structure of the synthesized diblock copolymer in the organic solvent THF was obtained by directly dissolving it in THF (the concentration of the polymer was 1 mg / mL) and stirring overnight at room temperature. The nano-aggregated structure of the copolymer in aqueous solution is obtained by dialysis. Specifically, 5 mg of the copolymer PEG-b-PPLG or PEG-b-(PPLG-g-R) were respectively dissolved in 1 mL of THF, and then 4 mL of deionized water was slowly added dropwise under stirring. After stirring overnight, THF was completely removed by dialysis in deionized water to obtain their self-assembled structures in aqueous solution.
[0059] by attaching Figure 4 The results of dynamic light dispersion (DLS) showed that block copolymers could form aggregates with a certain particle size in aqueous solution and THF, and had a narrow distribution range. This is th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com