Positive allosteric modulators of mGluR3
A technology of tautomers and solvates, applied in the field of substituted pyridine derivatives, can solve problems such as abnormal regulation of output activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
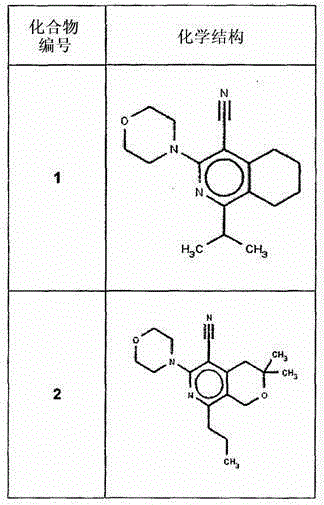
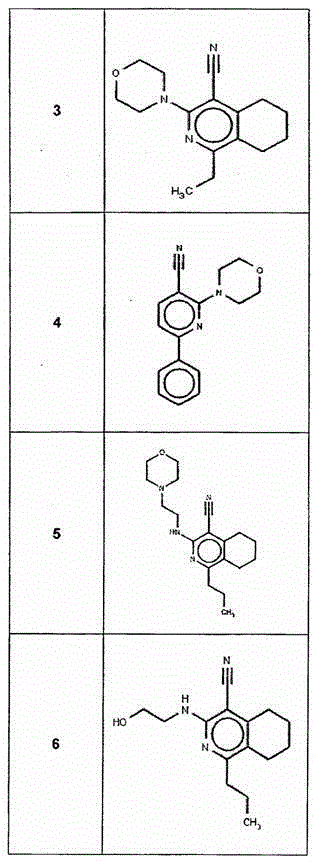
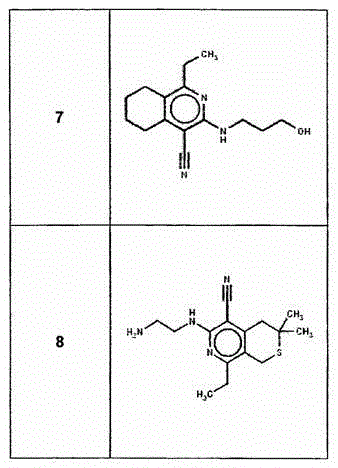
[0201] I. Synthesis of Selected Compounds of the Invention
[0202] The following compounds were synthesized and / or characterized. However, it is within the knowledge of those skilled in the art to prepare and / or characterize these compounds differently.
[0203] Step 1 - IS08641-042
[0204] 1-isopropyl-3-oxo-2,3,5,6,7,8-hexahydroisoquinoline-4-carbonitrile
[0205]
[0206] To a solution of 2-isobutyrylcyclohexanone (13 g, 0.0772 mol) in ethanol (250 mL) was added 2-cyanoacetamide (6.5 g, 0.0772 mol) and a catalytic amount of piperidine (3 mL ). After the reaction was complete (by LCMS), the precipitated solid was collected by filtration and dried under vacuum. This was slurried with ethyl acetate to give the title compound (10 g, 59%) as a white solid.
[0207] 1 H NMR (400MHz, DMSO-d 6 ) δ 11.87 (s, 1H), 3.17-3.10 (m, 1H), 2.74 (s, 2H), 2.50-2.47 (m, 2H), 1.66 (s, 4H), 1.19-1.17 (d, J = 7.0 Hz, 6H).
[0208] Step 2 - FS08641-051
[0209] 1-isopropyl-3-morphol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com