A kind of electrolytic preparation method of sulfur hexafluoride
A technology of sulfur hexafluoride and sulfur tetrafluoride, which is applied in the electrolysis process, electrolysis components, electrodes, etc., can solve the problems of easy adhesion of anode gas and drop in the conductive current of the electrolytic system, so as to prevent the sharp drop of the conductive current and ensure normal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1, preparation of nano-cerium oxide modified carbon electrode
[0023] In the reactor, add 100Kg nano-cerium oxide, 1200Kg (mass percentage concentration: 10) sulfuric acid, 0.3Kg 4-bromoboric acid diazonium tetrafluoroborate, mix for 30 minutes to prepare a suspension; put the carbon electrode into the suspension Soak in the liquid for 50 hours, then add 300Kg meta-porphyrin dimethyl ester, 0.3Kg cobalt acetate, soak for 60 hours at 50°C, take it out and dry it to prepare nano-cerium modified carbon electrode.
[0024] Step 2, the preparation of sulfur hexafluoride
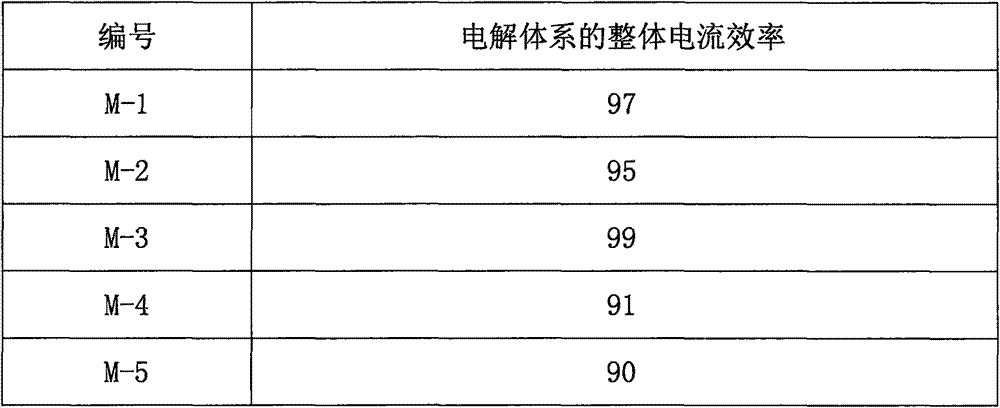
[0025] Sulfur tetrafluoride and hydrofluoric acid are made into an electrolyte in a molar ratio of 3:1 and continuously injected into the electrolytic cell. The anode of the electrolytic cell is the nano-cerium-modified carbon electrode in step 1. The electrolysis temperature is -50°C, the pressure is 3Mpa, and the voltage is 30V. Anode generated gas contains SF 6 , SF 4 As well as some trace impur...
Embodiment 2
[0027] Step 1, preparation of nano-cerium oxide modified carbon electrode
[0028] In the reactor, add 100Kg nanometer cerium oxide, 1000Kg (mass percent concentration: 5) sulfuric acid, 0.1Kg 4-bromoboric acid diazonium tetrafluoroborate, mix for 10 minutes to prepare a suspension; put the carbon electrode into the suspension Soak in the solution for 20 hours, then add 100Kg meta-porphyrin dimethyl ester, 0.1Kg cobalt acetate, soak for 20 hours at 20°C, take it out and dry it to prepare nano-cerium modified carbon electrode.
[0029] Step 2, the preparation of sulfur hexafluoride
[0030] Sulfur tetrafluoride and hydrofluoric acid are formulated as an electrolyte in a molar ratio of 1:1 and continuously injected into the electrolytic cell. The anode of the electrolytic cell is the nano-cerium-modified carbon electrode in step 1. The electrolysis temperature is -60°C, the pressure is 0.5Mpa, and the voltage is 20V. , the anode gas contains SF 6 , SF 4 As well as some trace ...
Embodiment 3
[0032] Step 1, preparation of nano-cerium oxide modified carbon electrode
[0033] In the reactor, add 100Kg nano-cerium oxide, 1500Kg (mass percentage concentration: 20) sulfuric acid, 0.5Kg 4-bromoboric acid diazonium tetrafluoroborate, mix for 50 minutes to prepare a suspension; put the carbon electrode into the suspension Soak in the solution for 80 hours, then add 500Kg meta-porphyrin dimethyl ester, 0.5Kg cobalt acetate, soak for 80 hours at 60°C, take it out and dry it to prepare nano-cerium modified carbon electrode.
[0034] Step 2, the preparation of sulfur hexafluoride
[0035] Sulfur tetrafluoride and hydrofluoric acid are made into an electrolyte in a molar ratio of 6:1 and continuously injected into the electrolytic cell. The anode of the electrolytic cell is the nano-cerium-modified carbon electrode in step 1. The electrolysis temperature is -30°C, the pressure is 5Mpa, and the voltage is 50V. Anode generated gas contains SF 6 , SF 4 As well as some trace imp...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap