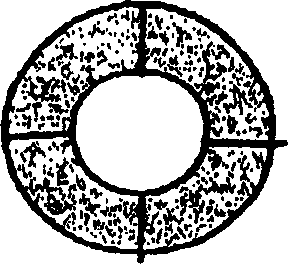
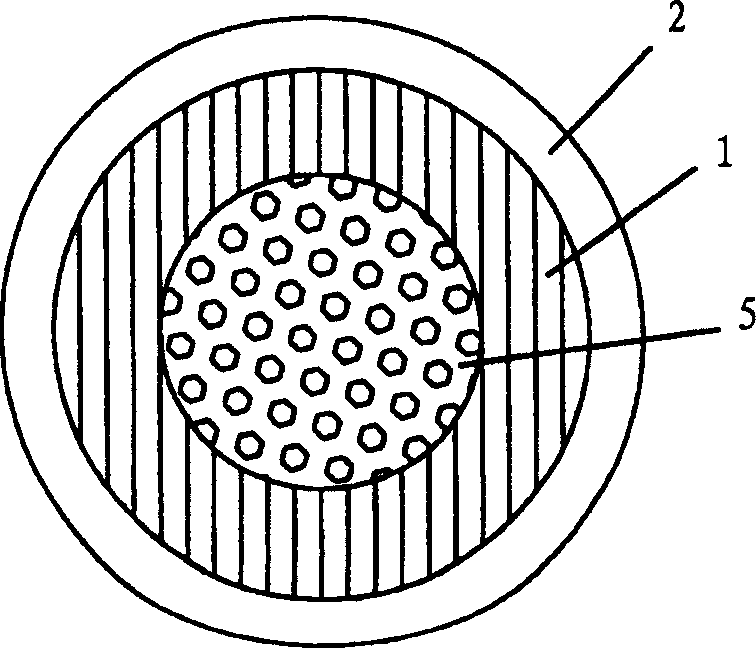
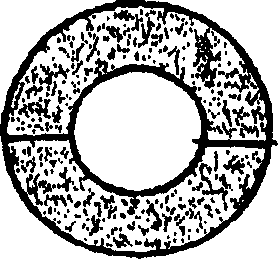
Pessary or intrauterine medicine release device containing antiestrogenic and anti-pregnant hormone composite preparation and its use
An anti-progestin and anti-estrogen technology, which can be used in medical preparations containing active ingredients, pharmaceutical formulations, and drug devices, etc. Side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Weigh 300 mg of mifepristone, 200 mg of letrozole, 0.1 gram of sodium lauryl sulfate, 0.1 gram of Span-20 and 0.7 gram of β-cyclodextrin (molecular weight 1134, produced by Shanghai Reagent Company), mix well Fill it into a silastic-382 medical silicone rubber tube with a wall thickness of 1 mm, put it into a molding mold, and heat press it.
Embodiment 2
[0070] Weigh a certain amount of HTV medical rubber (molecular weight 300,000-1,000,000, produced by Shanghai Rubber Products Research Institute) and extrude and vulcanize it into a medical rubber ring with a diameter of 5 cm. Weigh 300 milligrams of mifepristone, 200 milligrams of letrozole, 0.015 grams of Brij52 and 1.2 grams of HTV medical rubber (molecular weight 300,000-1,000,000, produced by Shanghai Rubber Products Research Institute), knead and press into a 1.2-mm-thick first Thin skin, this first thin skin is coated on the above-mentioned medical rubber ring that makes; Take again 200 milligrams of Letrozole, 0.01 gram of Brij52 and 1 gram of HTV medical rubber (molecular weight 30-1,000,000, produced by Shanghai Rubber Products Research Institute) ), after kneading, it is pressed into a second thin skin with a thickness of 1.2 mm, and the second thin skin is coated on the first thin skin, and the obtained article is coated with a 0.02 mm HTV medical silicone rubber th...
Embodiment 3
[0072] Obtain the medical silicone rubber liner tube of RTV-1 (molecular weight 0.74-110,000, Shanghai Rubber Products Research Institute) with a tube diameter of 4 mm by extrusion vulcanization molding, and bend the liner tube to enclose a diameter of 5 cm hollow circle.
[0073] Weigh 300 mg of HTV medical rubber (molecular weight 30-1 million, produced by Shanghai Rubber Products Research Institute), 400 mg of mifepristone, 0.3 gram of sodium lauryl sulfate, 0.1 gram of Span-80 and 0.8 gram of PEG1200, and weigh Get 200 mg of HTV medical rubber (molecular weight 30-1 million, produced by Shanghai Rubber Products Research Institute), 250 mg of letrozole, 0.1 gram of sodium lauryl sulfate, 0.05 gram of Span-80 and 0.3 gram of PEG1200, after they are mixed respectively Each was pressed into the first thin skin and the second thin skin, which were coated on the inner liner in turn, and then the RTV-1 medical silicone rubber was pressed into a thin skin with a thickness of 0.02 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com