Use of antiprogestins for the induction of apoptosis in a programming cell
An anti-progestin and cell death technology, which can be used in medical preparations containing active ingredients, anti-tumor drugs, organic active ingredients, etc., and can solve the problems of unobserved and substantial reduction of S-phase tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
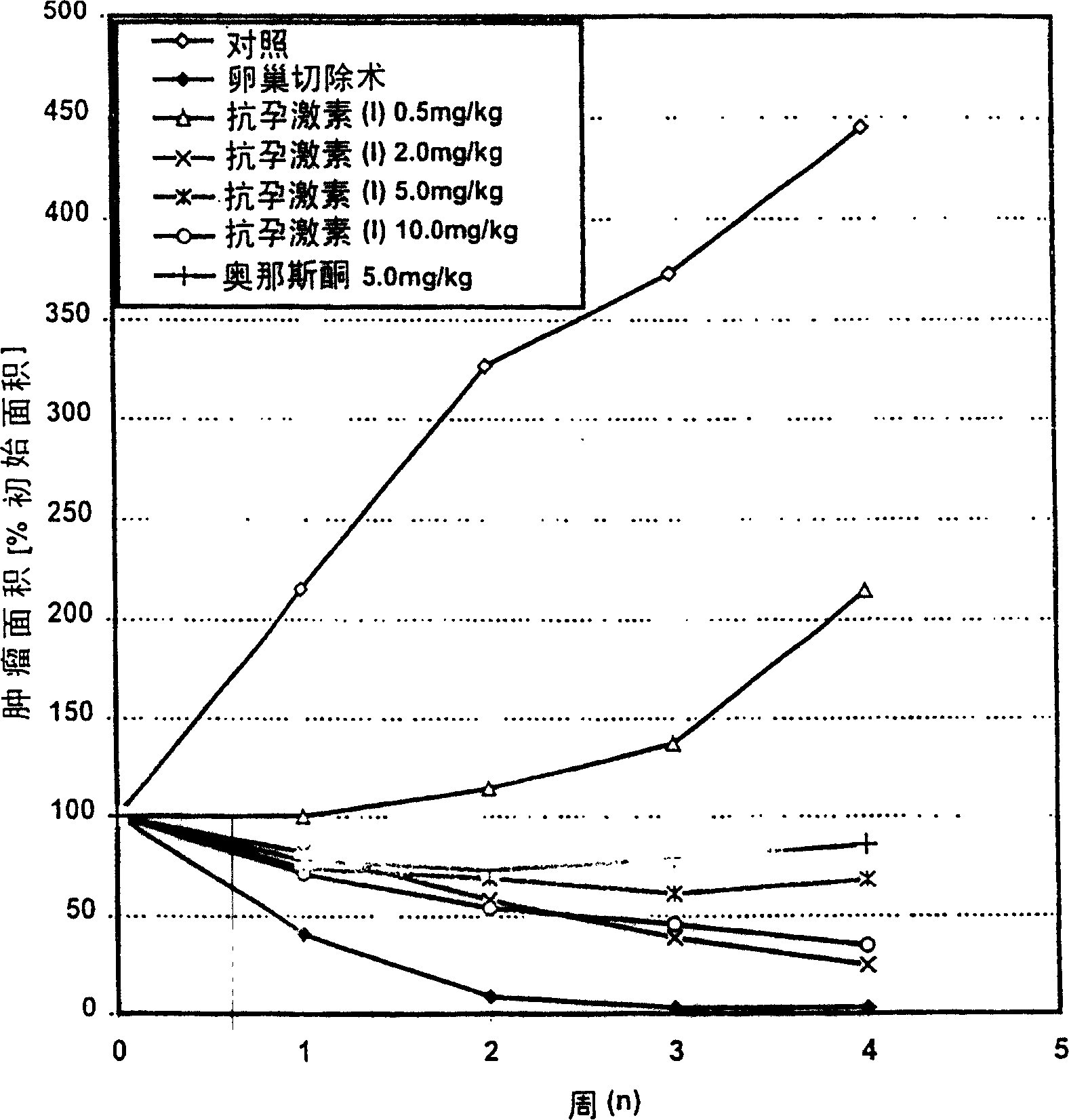
[0052] Example 1: Dose Response Study in DMBA-Induced Tumor Model
[0053] Materials and methods:
[0054] Immature female Sprague-Dawley rats (49-51 days old; 10 animals / group) were used in this study. Mammary gland tumors were induced by a single oral administration of 10 mg of 7,12-dimethylbenzanthracene (DMBA, Serva / Heidelberg). Will have at least one established tumor (greater than 150mm in size 2 ) rats were treated for 4 weeks by: 1) vehicle control, 2) ovariectomy at the beginning of treatment, 3) antiprogestogen (I), 0,5 mg / kg s.c., 4) antiprogestogen (I ), 2mg / kg s.c., 5) antiprogestogen (I), 5mg / kg s.c., 6) antiprogestogen (I), 10mg / kg s.c., and 7) onasitone, 5mg / kg, s.c., each day processing. The change in tumor area (percentage of initial tumor size) was measured weekly by calipers as a growth inhibition parameter. Statistical analysis of group differences in means was performed using the Kruskal Wallis-test. Further elaboration and evaluation of the DMBA pr...
Embodiment 2
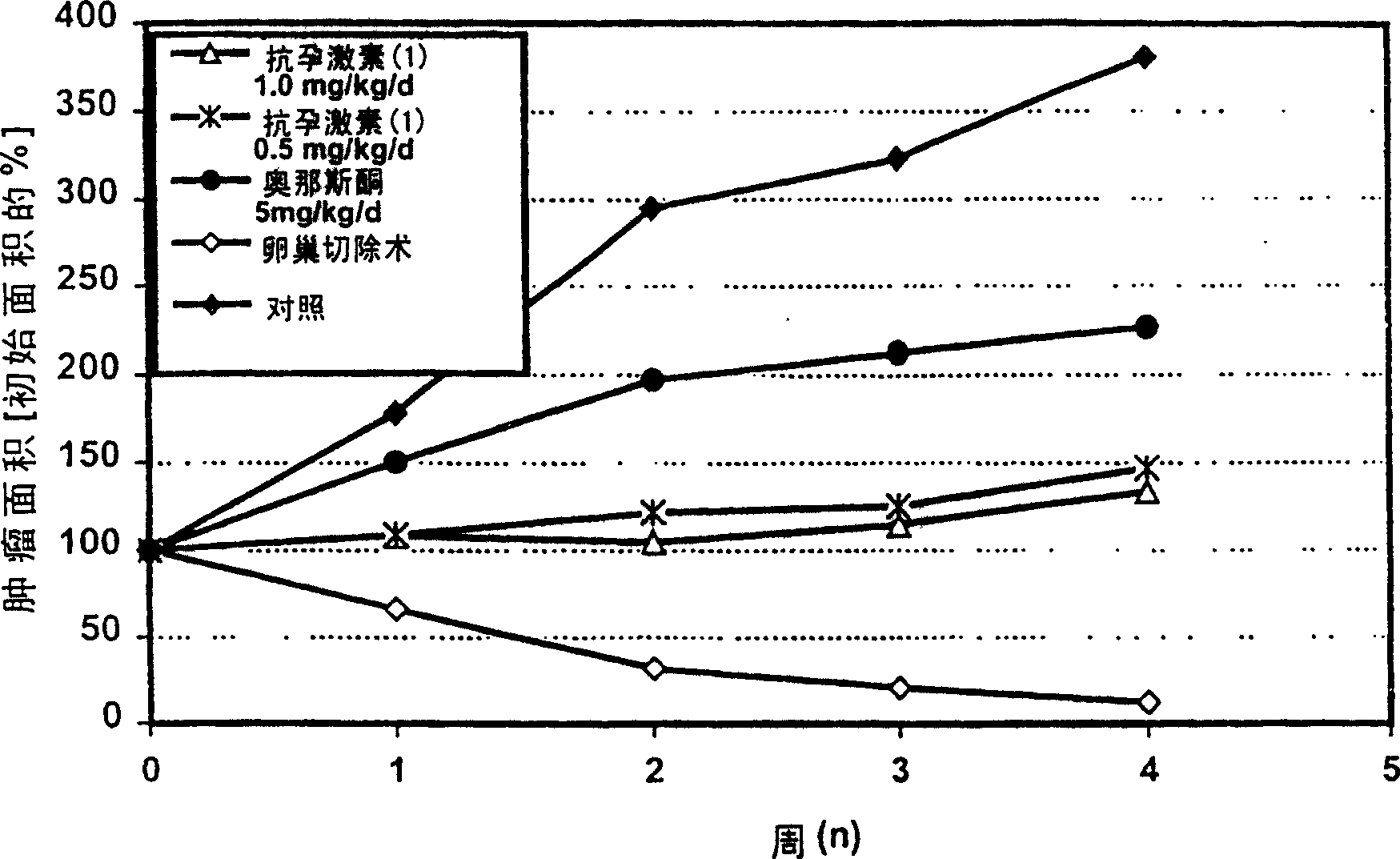
[0059] Example 2: Tumor Growth Inhibition in a Rat NMU-Induced Breast Cancer Model
[0060] Materials and methods:
[0061] Tumors were induced in female Sprague-Dawley rats (obtained from Tierzucht Schönwalde, 50-55 days old) by a single intravenous injection of NMU (nitrosomethylurea, 50 mg / kg). Beginning after 10 days, rats with at least one established tumor were treated for 4 weeks by: 1) vehicle control, 2) ovariectomy at the beginning of treatment, 3) antiprogestin (I), 1.0 mg / kg / day, 4) antiprogestin (I), 0.5mg / kg / day, and 5) onasirdone, 5mg / kg / day. The change in tumor area (percentage of initial tumor size) was measured weekly by calipers as a growth inhibition parameter. Statistical analysis of group differences in means was performed using the Kruskal Wallis-test.
[0062] result:
[0063] In intact control animals, further tumor growth was observed, whereas ovariectomy resulted in complete tumor growth inhibition. Treatment with antiprogestins (I) at doses of...
Embodiment 3
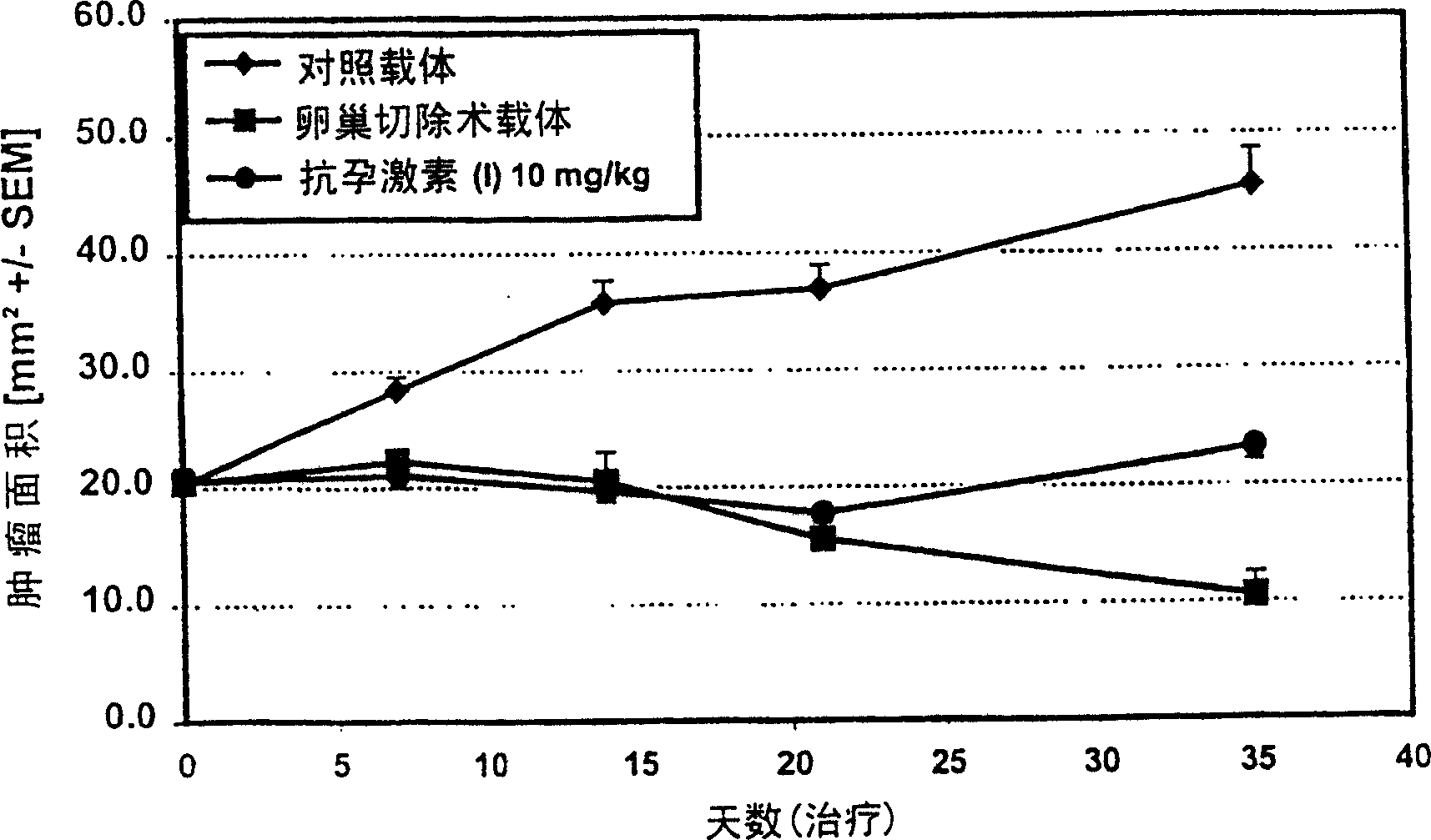
[0066] Example 3: Human T47D breast cancer xenograft in scid mice
[0067] Materials and methods:
[0068] Female Fox Chase scid mice (M & B) were supplemented with estradiol pellets (Innovative Research of America). T47D breast cancer cells obtained from cell culture and suspended in matrigel were implanted into the inguinal region of mice. When the tumor size is about 25mm 2 , start treatment. Treatment is continued until the tumor is suppressed. The experimental groups were: 1) control group (vehicle), 2) ovariectomy, 3) antiprogestin (I), 10 mg / kg s.c. Tumor size was determined with calipers. Kruskal Wallis-test was used for statistical analysis of mean group differences.
[0069] result:
[0070] In the T47D breast cancer model, in contrast to the rapid tumor growth in the control group, ovariectomy caused significant inhibition of tumor growth. image 3 It is clearly shown that s.c. application of 10 mg / kg antiprogestin (I) induces apoptosis in tumor cells. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com