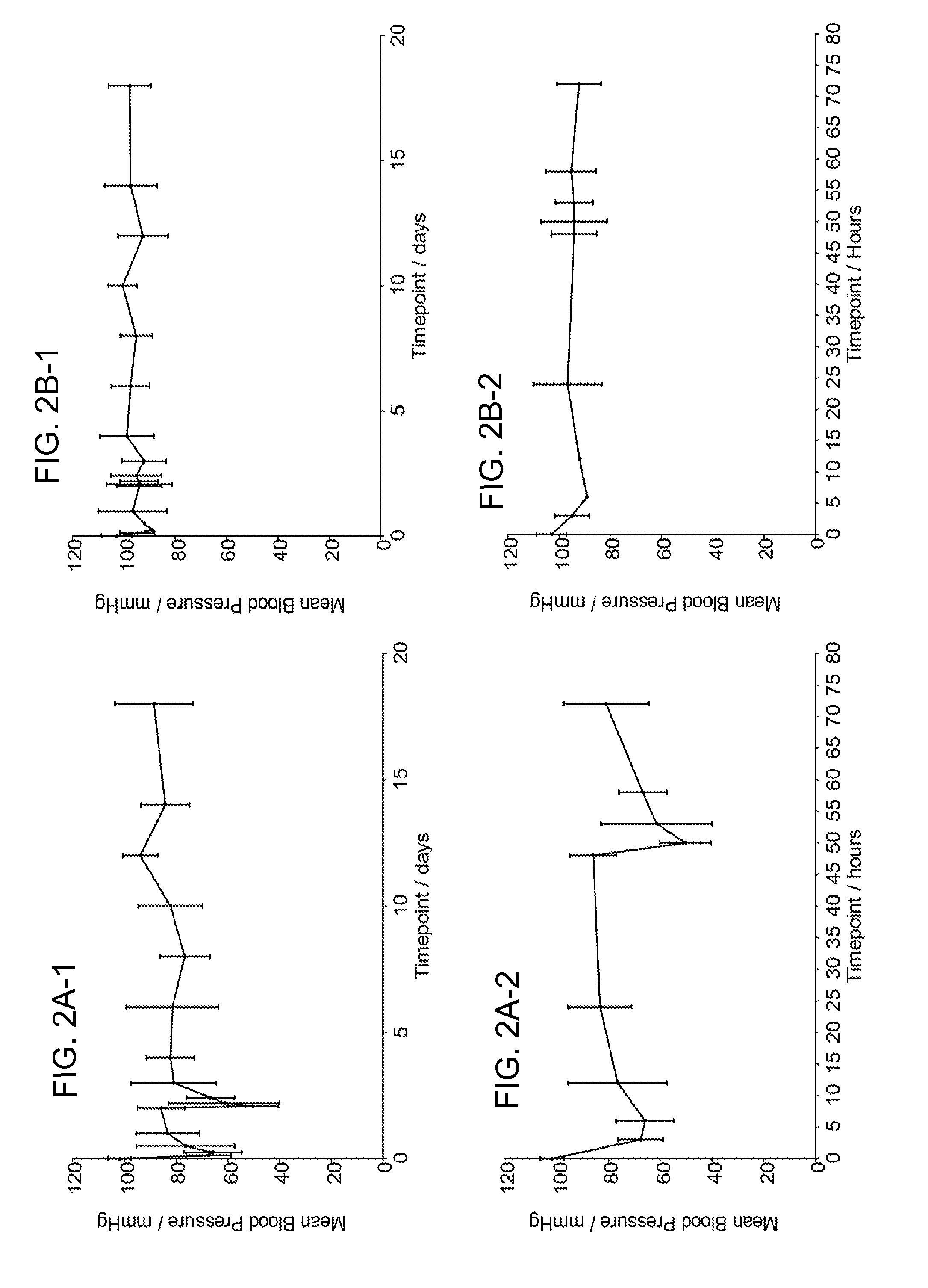Natriuretic polypeptide delivery systems
a delivery system and natriuretic polypeptide technology, applied in the direction of peptides, peptide/protein ingredients, chemistry apparatus and processes, etc., can solve the problems of high occurrence of re-admission and mortality rate associated with discharged patients, and achieve the effect of improving hf symptoms, high occurrence of re-admission and mortality ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sustained Delivery of Natriuretic Polypeptides for Three Weeks with In Situ Polymer Precipitation Delivery System
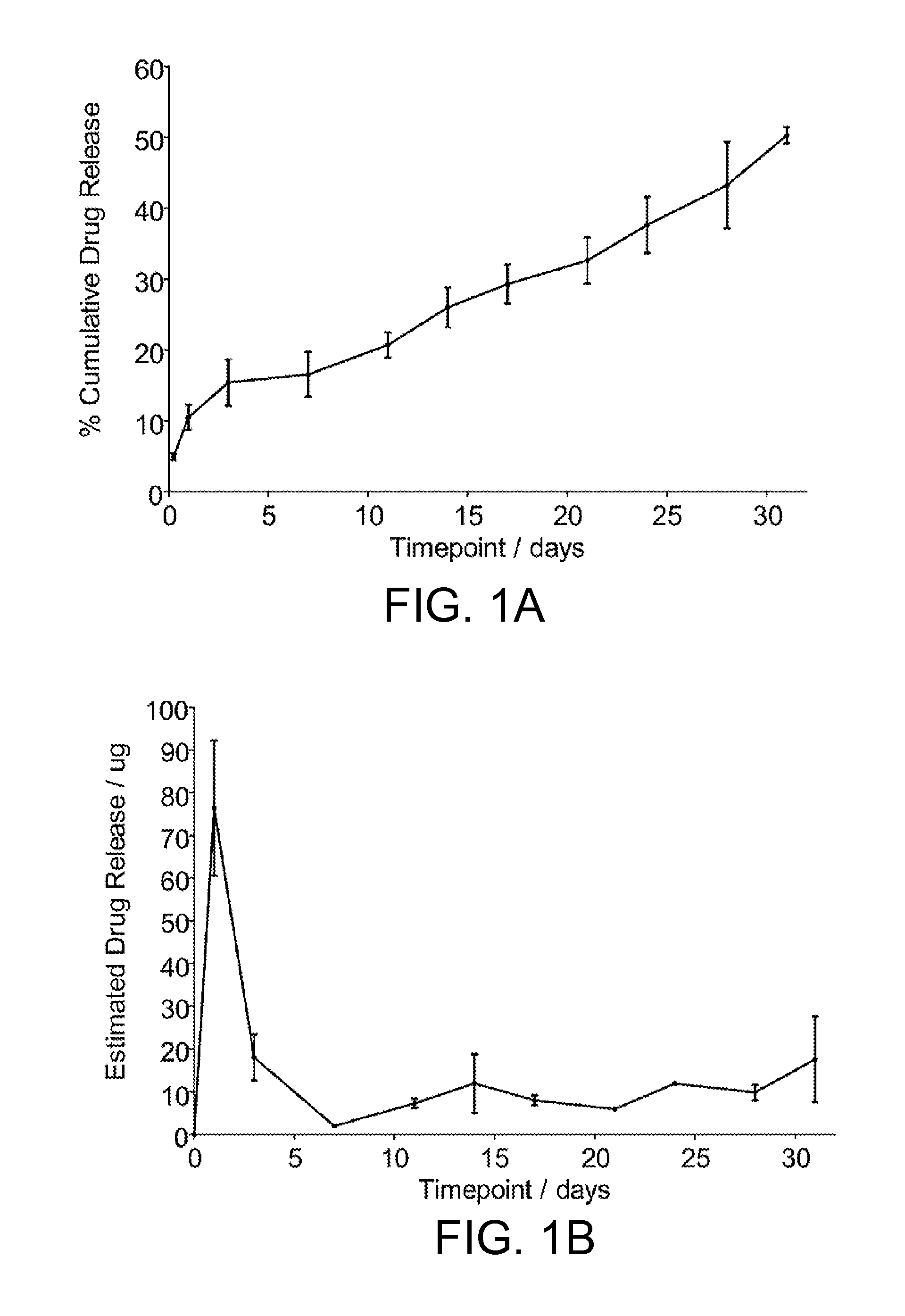
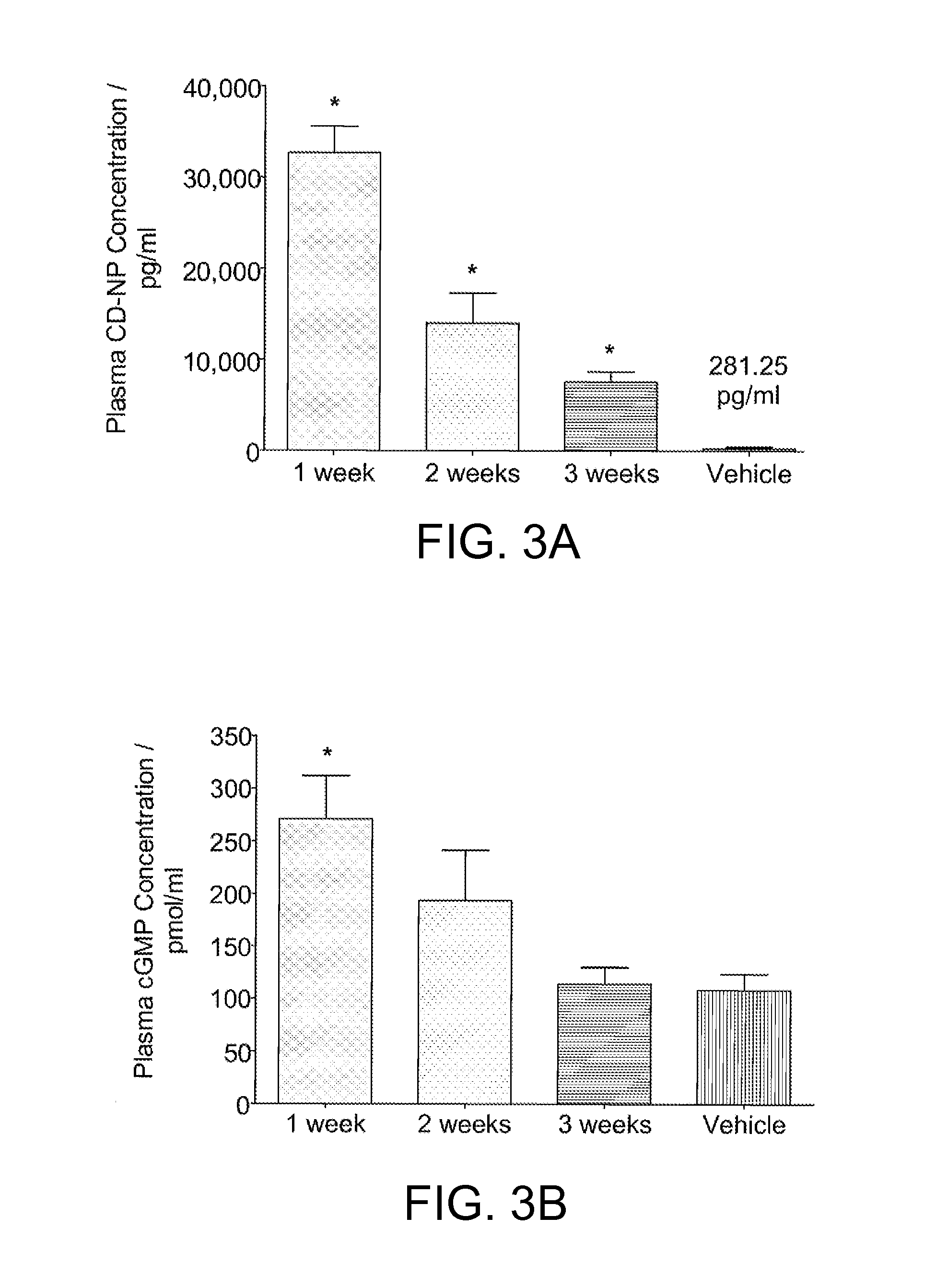
[0036]0.45% percentage weight / weight (w / w) of CD-NP was mixed with 40% poly(lactic-co-glycolic acid) in 39.55% (w / w) N-methyl-2-pyrrolidinone and 20% (w / w) triacetin. The resulting mixture was allowed to homogenize overnight. Three groups of 5 rats (Wistar male, 250-300 g) were injected subcutaneously with the gel. A fourth group (n=5) was injected with blank gels as vehicles. Rats sacrificed at respective time points (1 week, 2 weeks, or 3 weeks) for plasma and urinary evaluation.
[0037]Plasma CD-NP levels were significantly higher than vehicle: 32,700±2888 pg / mL, 13,977±3302 pg / mL, and 7,566±1115 pg / mL at weeks 1, 2, and 3 after gel injection. 24-hour urinary CD-NP excretion levels were significantly elevated at 107.3±12.7 pg / minute, 33.7±29.7 pg / minute, and 16.5±8.2 pg / minute at weeks 1, 2, and 3 as compared to 2.02±0.10 pg / minute pre-injection. No significant differenc...
example 2
In Vivo Evaluation of an In Situ Polymer Precipitation Delivery System for Natriuretic Polypeptides
Materials
[0039]Poly D,L-lactic-co-glycolic acid (PLGA) (17 kDa, 50:50 dl-LA to GA ratio, inherent viscosity: 0.2 dL / g; obtained from Purac Biomaterials, Gorinchem, Netherlands) was used to form gel formulations. HPLC grade N-methyl-2-pyrrolidinone (NMP; obtained from Sigma Aldrich) and triacetin (obtained from Fisher Scientific) were used as solvents. Both solvents were of low toxicity. CD-NP polypeptide preparations were obtained from Nile Therapeutics, Inc.
Synthesis of In Situ Polymer Precipitation Delivery System
[0040]Preparation of the injectable gel formulation was as followed. Briefly, CD-NP was dissolved in NMP solvent, and the resulting suspension was allowed to stir for at least 3 to 4 hours before the addition of pre-weighed polymer and triacetin solution. The quantities added were such that the polymer and triacetin content was 40% and 20% by weight, respectively, with the C...
example 3
Natriuretic Polypeptide Release Properties
[0072]In vitro studies were performed to determine the release properties of CU-NP polypeptides from polymer gels. An injectable gel system was designed and used to evaluate sustained release of CU-NP polypeptides over one month. Several gel parameters were investigated. First, three types of polymers were investigated: PLGA (50 / 50) with an intrinsic viscosity 0.4 dL / g (IV0.4), PLGA (50 / 50) with an intrinsic viscosity of 0.2 dL / g (IV0.2), and acid-capped PLGA (50 / 50) with an intrinsic viscosity of 0.2 dL / g (IV0.2A). Second, three different percentages of polymer within the gel preparation were investigated: 20 percent polymer (PGLA), 30 percent polymer (PGLA), and 40 percent polymer (PGLA). Third, different levels of triacetin solvent were evaluated: zero percent triacetin, 10 percent triacetin, and 20 percent triacetin. Fourth, two drug loading concentrations were tested: 0.15 percent CU-NP polypeptides and 0.3 percent CU-NP polypeptides. A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com