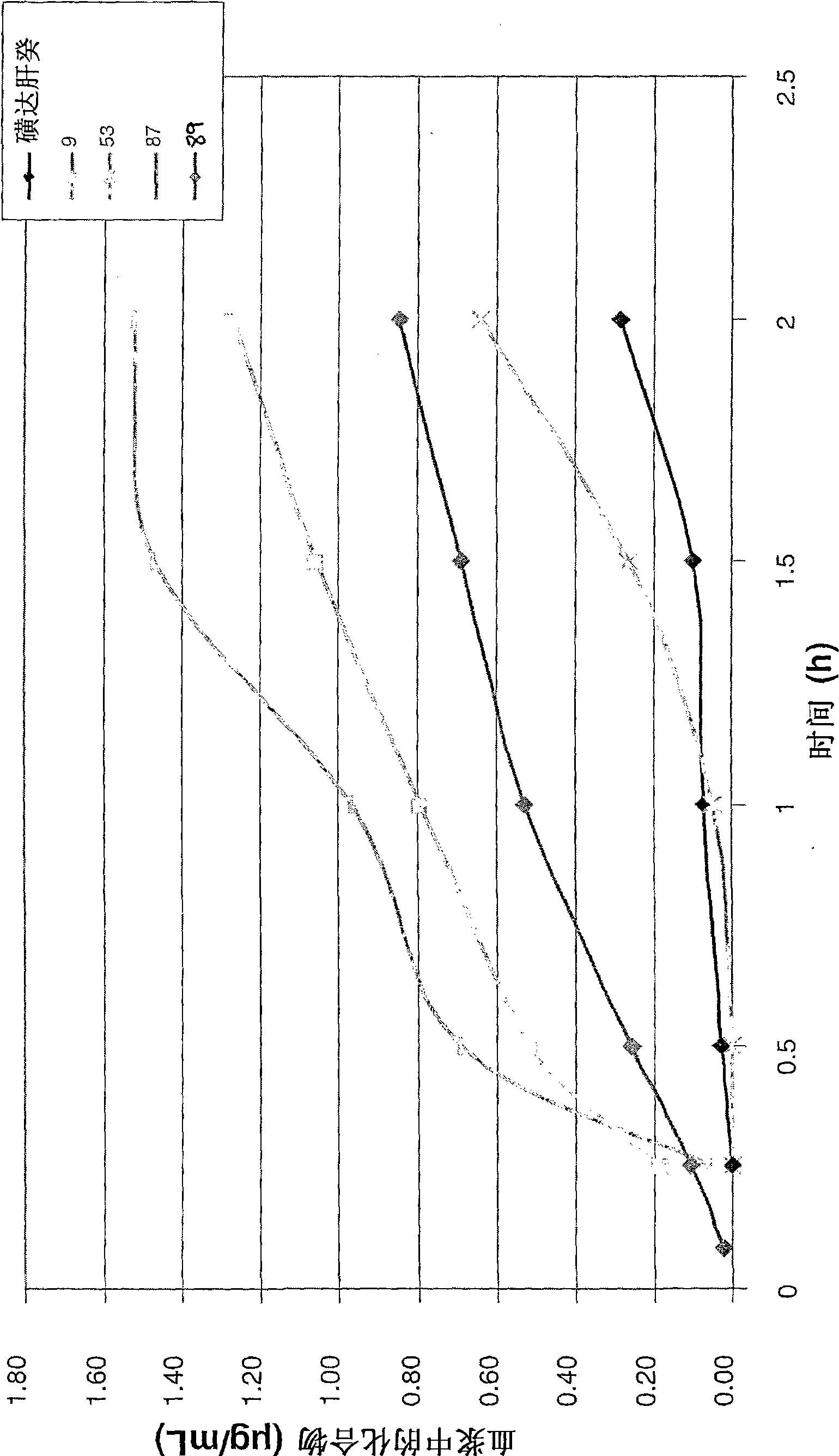
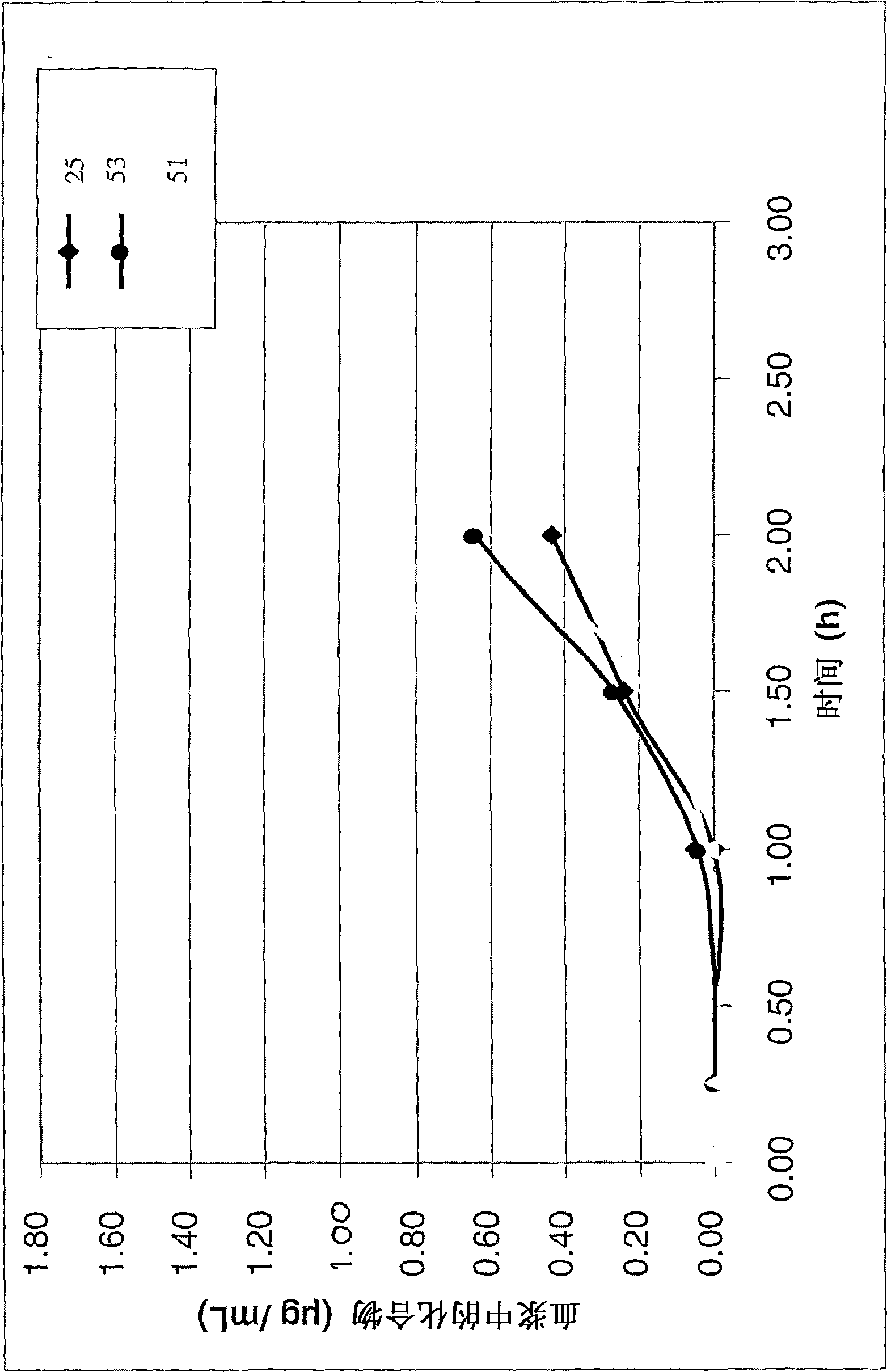
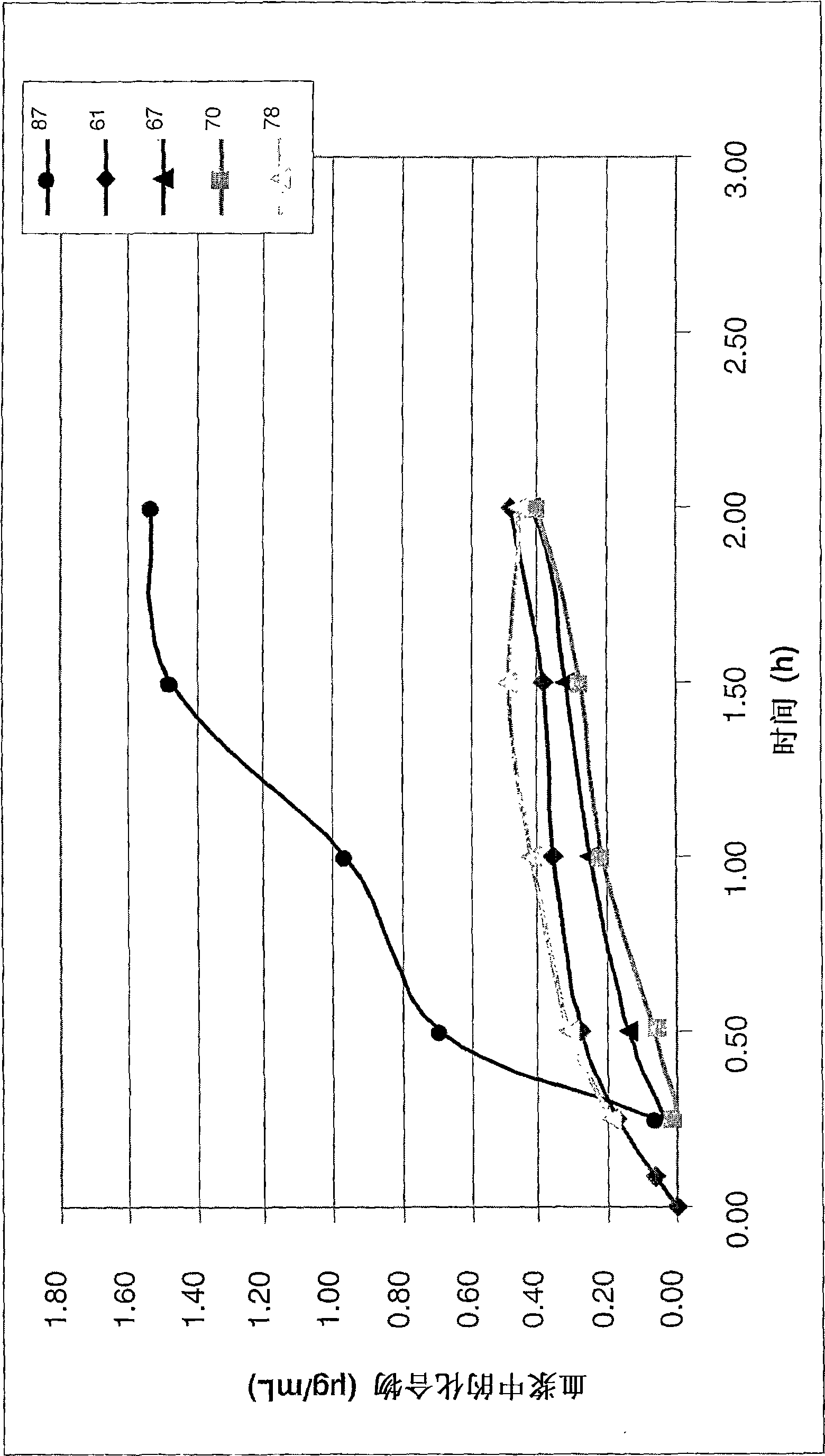
Anticoagulant compounds
A compound and solvate technology, applied in the field of anticoagulant oligosaccharides, can solve problems such as decreased bioavailability and inability to absorb
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0588] This example was prepared from Example 2 according to method T (yield: 39%).
[0589] 1 H NMR (400MHz, CD 3 OD, ppm), δ: 5.46-5.38 (width s, 1H, H-1), 5.00-4.84 (m, 3H, H-1).
[0590] ESI-MS, negative mode, m / z: 925.7 [M+2DBA-4H] 2- , 861.1 [M+DBA-3H] 2- , 796.5 [M-2H] 2-
Embodiment 2
[0592] This example was prepared according to method P (yield: 91%).
[0593] 1 H NMR (400MHz, CD 3 OD, ppm), δ: 5.40 (d, 1H, J=3.6Hz, H-1Glc III ), 5.18 (d, 1H, J=3.4Hz, H-1Glc V ), 4.96(s, 1H, H-1ManUA II ), 4.92 (d, 1H, J=3.4Hz, H-1Glc I ), 4.77 (1H, H-1Glc IV ).
[0594] ESI-MS, negative mode, m / z: 1061.2 [M+2DBA-4H] 2- , 996.6[M+DBA-3H] 2- .
Embodiment 3
[0596] This example was prepared according to method P (yield: 85%).
[0597] 1 H NMR (400MHz, CD 3 OD, ppm), δ: 5.39-5.34 (wide s, 1H, H-1), 5.15 (d, 1H, J=3.2Hz, H-1), 5.00 (s, 1H, H-1), 4.94- 4.87 (m, 2H, H-1), 4.72 (1H, H-1).
[0598] ESI-MS, negative mode, m / z: 1061.2 [M+2DBA-4H] 2- , 996.6[M+DBA-3H] 2- .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com