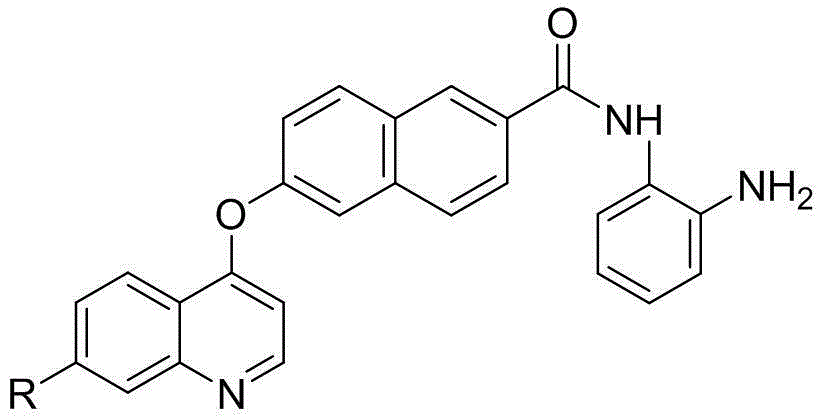
Naphthlamide derivative used as protein kinase inhibitor and histone deacetylase inhibitor and preparation method of naphthlamide derivative
A technology of protein kinase inhibitor and sirtuin, which is applied in the field of preparation of naphthalene amide derivatives, can solve the problems of large pollution, high price, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
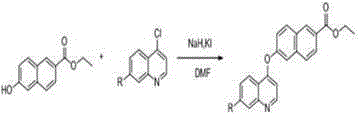
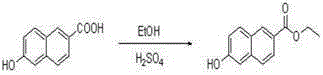
[0030] Dissolve 1.88 g (10 mmol) of compound 6-hydroxynaphthalic acid in 80 ml of ethanol, add 4 drops of concentrated sulfuric acid, heat and reflux at 80 ℃ for 5 hours, then remove the solvent under reduced pressure to obtain compound 6-hydroxynaphthalate ethyl Ester 1.98 g (9.2 mmol), yield 91.7%; compound 1.98 g (9.2 mmol) ethyl 6-hydroxynaphthalate was dissolved in 10 ml DMF, stirred under ice bath, slowly added dropwise containing 0.52 g (21.7 mmol) ) NaH in DMF solution, stir for half an hour in an ice bath, remove the ice bath, wait for the temperature to return to room temperature, slowly drop in 2.19 g (11.1 mmol) 4,7-dichloroquinoline, and 0.3 g (1.8 mmol) 10 ml of DMF solution of KI, stirred for half an hour, reacted at 11 ℃ for 8 hours, then removed the solvent under reduced pressure to obtain the reaction mixture, separated by silica gel column chromatography, the gradient of the mobile phase was: V (n-hexane): V (ethyl acetate Ester)=4.5:1 to obtain compound 6-(7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com