Immunogenic fusion protein for tuberculosis and application of immunogenic fusion protein
A fusion protein, immunogenic technology, applied in the field of fusion proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
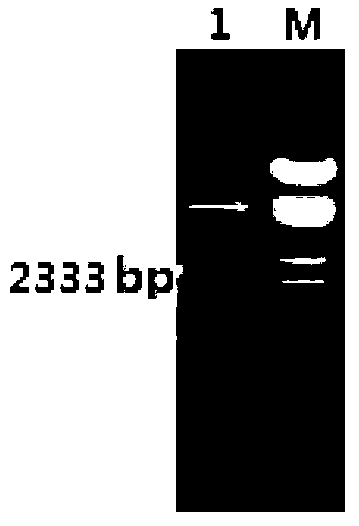
[0125] The construction of embodiment 1 genetically engineered bacteria
[0126] 1.1 Design of primers
[0127] According to the Ag85a and Rv2626c sequences of the genomic DNA of the standard Mycobacterium tuberculosis strain H37Rv, a pair of specific primers were designed respectively. The primers were synthesized by Beijing Liuhe Huada Gene, and the primers were as follows:
[0128] Ag85aF: 5'-ATAAAGAAAATTCAATTTCATTTTCCCGGCCGGGCTTG-3' (SEQ ID NO: 12)
[0129] Ag85aR: 5'-AATGCT GGATCC GCCACCGCCGGCGCCCTGGGGCGCGGGCA-3' (SEQ ID NO: 13)
[0130] Note: The underline represents the restriction site of BamH I
[0131] Rv2626cF: 5'-ATT GGATCC GGTGGCGGTGGCTCCACCACCGCACGCACAT-3' (SEQ ID NO: 14)
[0132] Note: The underline represents the restriction site of BamH I
[0133] Rv2626cR: 5'-CGTGTTTCTTTTCGATTGGCTGGCGAGGGCATG-3' (SEQ ID NO: 15)
[0134] According to the LLO upstream and downstream sequences of the genomic DNA of Listeria monocytogenes yzuLM4, a pair of specific primers...
Embodiment 2
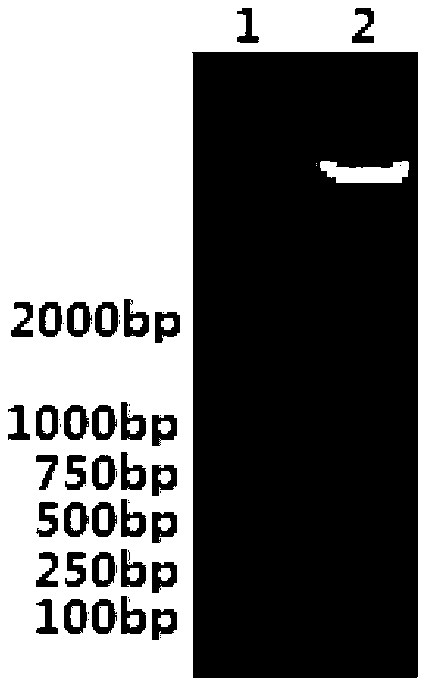
[0182] Example 2 Detection of the expression of the target gene at the protein level
[0183] Inoculate the LMΔhly::Ag85a-Rv2626c constructed in Example 1 into the BHI liquid medium, culture on a shaker at 37°C for 15 hours, take 1ml of the bacterial liquid, centrifuge to remove the bacterial cells, and collect the culture supernatant. The secreted protein obtained by the TCA precipitation method was subjected to SDS-PAGE electrophoresis. After the electrophoresis, the gel was taken out, the length and width were measured, and the NC membrane was cut out 0.5 cm shorter than the length and width of the gel. The length and width of the gel are 1cm shorter filter paper, soak them in the transfer buffer, and transfer them to the electrotransfer device according to the structure of filter paper-filter paper-gel-NC membrane-filter paper-filter paper, after the transfer is completed, take out the NC The membrane was placed in 1% BSA-PBS blocking solution and blocked overnight. Wash ...
Embodiment 3
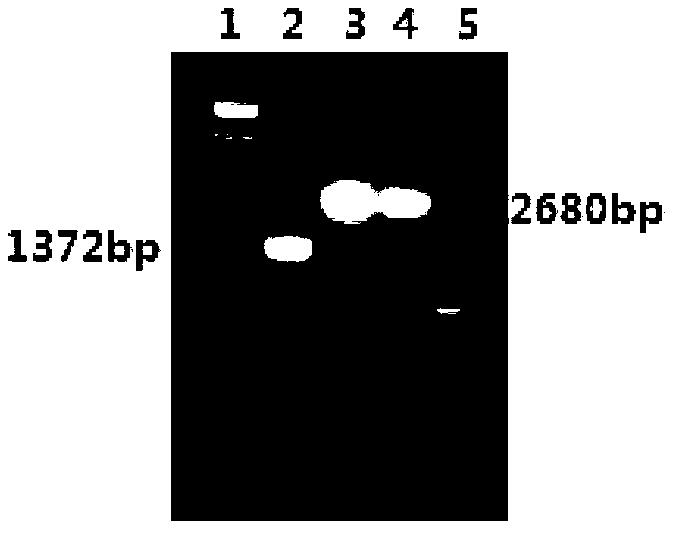
[0198]Example 3 Safety Evaluation of Recombinant Bacteria
[0199] 3.1 Hemolytic activity test of secreted fusion protein LLO-Ag85a-Rv2626c
[0200] Inoculate the LMΔhly::Ag85a-Rv2626c and yzLM4 constructed in Example 1 to a quantitative volume of BHI liquid medium, culture on a shaker at 37°C for 15 hours, take 1ml of the bacterial liquid, centrifuge to remove the bacterial cells, and collect the culture supernatant. After adjusting the OD600 of the supernatant to the same size, take 60 μl of the supernatant and dilute it with PBS (pH6.0) from the stock solution to 27 equal times, add it to the V-shaped 96-well hemagglutination plate, and then add it to each well 20 μL of 1% goat erythrocyte suspension, mixed evenly, placed in a 37°C incubator for 1 hour to observe the hemolysis, and the dilution of the well where 50% of the erythrocytes were hemolyzed was judged as the hemolysis titer. PBS was used as the control group. see results Figure 6 .
[0201] The results showed...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap