Method for improving cell transfection efficiency through utilization of bacterial artificial chromosome homologous recombination
An artificial chromosome and homologous recombination technology, applied in the direction of recombinant DNA technology, the use of vectors to introduce foreign genetic material, etc., can solve problems such as troublesome operation, large BAC, and difficult molecular operation, and achieve good results and improve efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1L
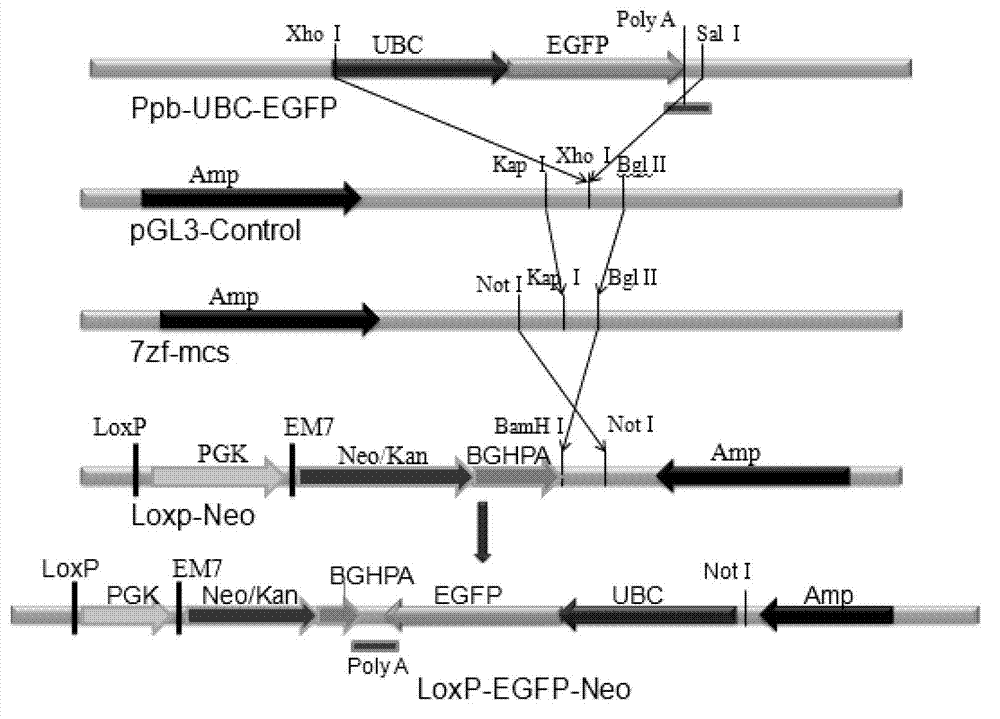
[0024] Example 1 Construction of LoxP-eGFP-neo vector
[0025]1. Cut out the pPB-eGFP-neo plasmid (Wellcome Trust Sanger) with a Loxp site and eGFP (enhanced Green Fluorescent Protein, enhanced green fluorescent protein) eukaryotic expression regulatory elements from pPB-eGFP-neo plasmid (Wellcome Trust Sanger) with xho and sal The complete sequence was connected to the PGL3-contro (purchased from PROMEGA, E1741) vector digested with xho to construct a PGL3-loxp-eGFP vector.
[0026] 2. Digest the PGL3-loxp-eGFP plasmid with Bgl and Kpn, cut off the Loxp-eGFP fragment with Bgl and Kpn cohesive ends, and connect it to the 7zf-mcs (in the PROMEGA company) that has been digested with Bgl and Kpn On the basis of the pGEM-7zf vector, the cloning site was changed to obtain the 7zf-mcs vector), and the 7zf-loxp-eGFP vector was constructed.
[0027] 3. Digest the 7zf-loxp-EGFP vector with Bgl and not, cut out the Loxp-eGFP fragment with Bgl and not cohesive ends, and connect it to th...
Embodiment 2
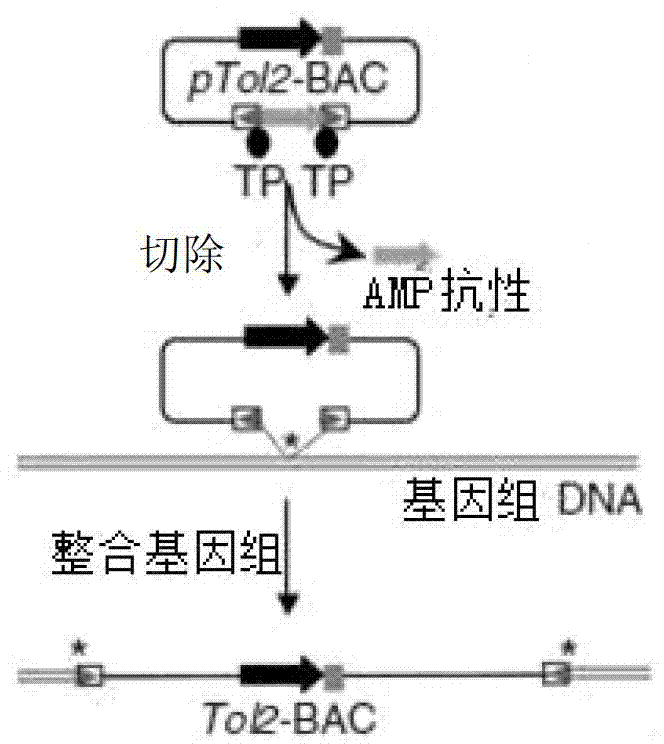
[0029] Construction of embodiment 2pCC1BAC-Tol2 vector
[0030] 1. Three correctly identified BAC clones (numbered 706H15, 169D05, 572K17) were prepared to be electroshock competent.
[0031] 2. Transform the pABRG recombinant plasmid into electric shock-competent three BACs (numbered 706H15, 169D05 and 572K17) and identify them.
[0032] 3. Prepare the above-identified positive clones into BAC electric shock competent.
[0033] 4. Design homology arm primers, PCR amplify the Tol2 transposable element (SEQ ID No.5), and recover the fragments amplified by PCR.
[0034] 5. Electric shock the recovered PCR fragments into BAC electric shock competent.
[0035] 6. Pick a single clone for PCR and sequencing identification, and save the strain after identification is correct.
[0036] 7. Prepare correctly identified pCC1BAC-Tol2 positive clones to be electroshock-competent for recombination.
[0037] There are 3 different BAC clone numbers, and the pCC1BAC-Tol2 vectors are respec...
Embodiment 3
[0039] Example 3 Construction of plasmid pCC1BAC-Tol2-eGFP
[0040] 1. Prepare the correctly identified pCC1BAC-Tol2 positive clones to be electroshock-competent containing the recombinant plasmid pABRG.
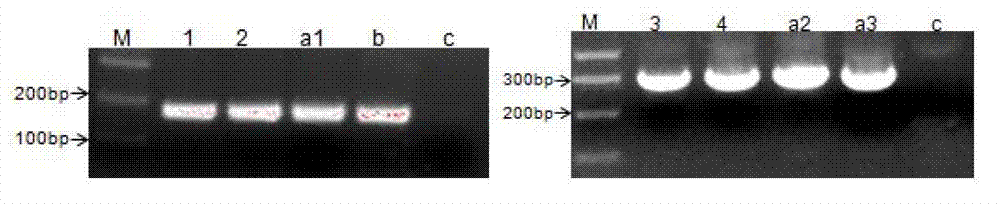
[0041] 2. Design homology arm primers, PCR amplify the sequence fragments carrying LoxP-eGFP-neo in the LoxP-eGFP-neo vector, and recover the PCR-amplified fragments.
[0042] 3. Transfer the recovered PCR fragment into the electroshock-competent pCC1BAC-Tol2 prepared above containing the recombinant plasmid pABRG by electric shock.
[0043] 4. Pick positive clones for cross-fragment PCR and sequencing identification, and save the strain after identification is correct.
[0044] 5. The firm and correct positive clones were extracted without endotoxin and prepared for electrotransfection.
[0045] Similarly, the vectors were labeled as 706H15-Tol2-eGFP, 169D05-Tol2-eGFP and 572K17-Tol2-eGFP vectors, and the sequences are shown in SEQ ID No.3, SEQ ID No.8 and 9, respectively...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com