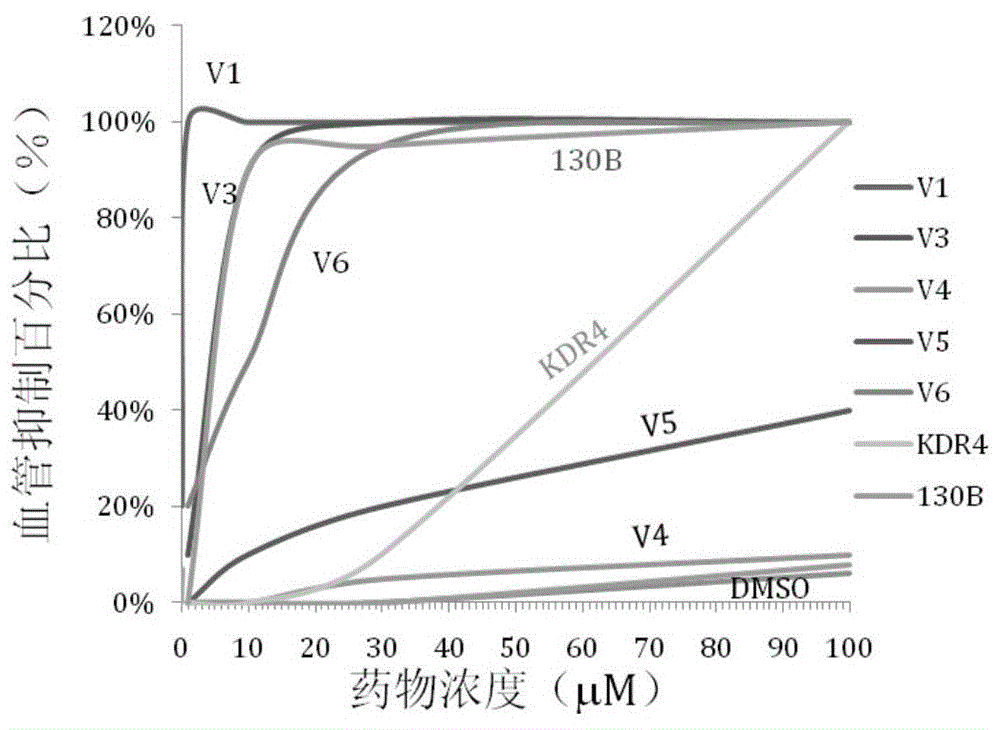
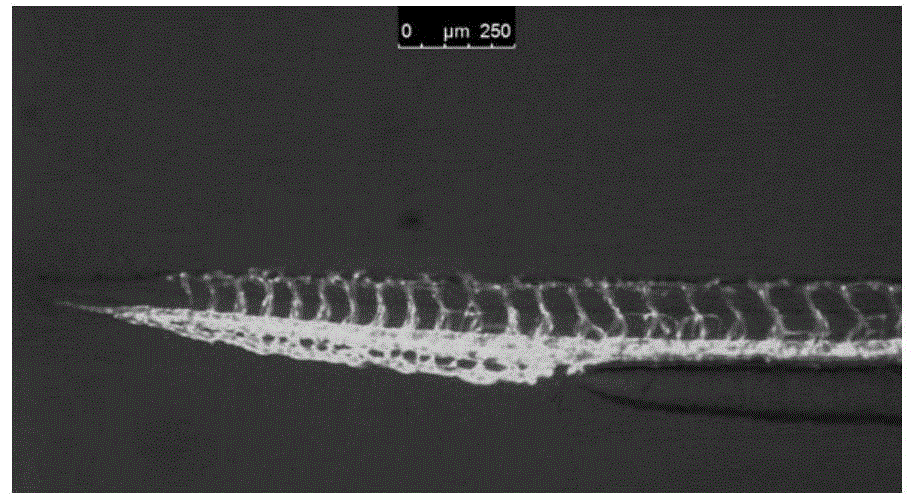
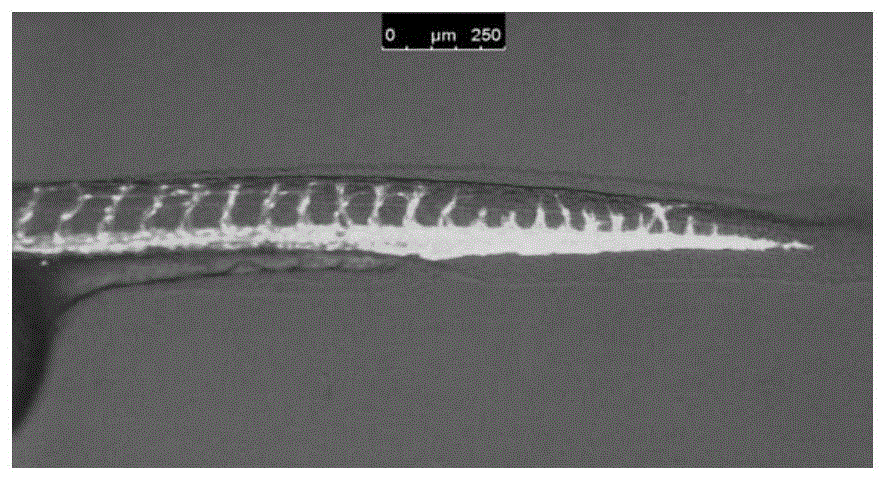
An anti-angiogenic compound
An angiogenesis, compound technology, applied in cardiovascular system diseases, organic chemistry, drug combination, etc., can solve the problem of high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 intermediate of the present invention
[0068]
[0069] Proceed as follows:
[0070] Step 1: Mix Michaelis acid (75.3 g, 0.522 mol) and triethyl orthoformate (92 mL, 0.55 mol), heat to 55°C for 90 minutes, then cool to 45°C. Compound 1 (92g, 0.5mol) was dissolved in methanol (200mL), and added to the reaction mixture to react for 45 minutes while keeping the temperature of the reaction mixture below 50°C, then stirred overnight at room temperature, thin layer chromatography (PE / EA =3:1) Monitor until the reaction is complete. The reaction mixture was cooled to 0°C, and the precipitate was filtered and dried to obtain pure compound 2 (155.9 g, yield: 96%) as a white solid.
[0071] Step 2: Compound 2 (155.9g, 0.441mol) was heated to 100°C in a Dowtherm A heat pipe (1.1L), and then slowly added to a flask connected to a Dowtherm A heat pipe (500mL, preheated to 210 °C), while maintaining the temperature above 207 °C. The reactants we...
Embodiment 2
[0079] Preparation of Example 2 Compound V01 (also denoted as V01 in the present invention)
[0080] step 1:
[0081]
[0082] Compound 11A (20 g, 0.15 mol), DMAP (1.9 g, 15.4 mmol) and (BoC) 2 O (75 g, 0.34 mol) was mixed in tetrahydrofuran (750 mL), and the reaction mixture was stirred at room temperature overnight. Thin layer chromatography (PE / EA=3:1) showed that the reaction was complete. The reaction mixture was concentrated, suspended in PE / EA (10:1, 200 mL), and filtered to obtain pure compound 11 (50 g, 100%) as a white solid.
[0083] Step 2:
[0084]
[0085] Mix compound 10 (50 mg, 0.15 mmol) and Cs2CO3 (150 mg, 0.45 mmol) in DMSO (1 mL), stir at room temperature under N2 for half an hour, then add compound 11 (60 mg). The resulting reaction mixture was stirred for half an hour and was detected by thin layer chromatography (DCM / methanol=10:1) until the reaction was complete. The reaction mixture was diluted with water, extracted with EA, the organic phas...
Embodiment 3
[0087] Preparation of Example 3 Compound V3 (also denoted as V03 in the present invention)
[0088] step 1:
[0089]
[0090] Stir and mix compound 13 (5.0g, 45mmol) and pyridine (200mL), and (Boc) 2 O (14.7 g, 67.5 mmol) was added dropwise to the above mixture. After the addition, the reaction was stirred at 85 °C for 4 hours. Thin-layer chromatography (DCM / methanol=10:1) tracked to the completion of the reaction, cooled the reaction mixture to 0 °C, added concentrated HCl (100 mL) and H2O (50 mL), after EA extraction, collected the organic phase and washed it with NaHCO3 aqueous solution , after drying over Na2SO4, a yellow oil was obtained, which was suspended in Et2O and filtered to obtain white solid compound 14 (4.3 g, yield: 45.2%).
[0091] Stir and mix compound 14 (2.4g, 11.4mmol) and N,N dimethylaniline (6.6mL) in DCM (84mL), add POCl dropwise at 0°C under N2 3 (3.2 mL, 34.2 mmol). After the addition, the reaction mixture was stirred at room temperature for 2...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap