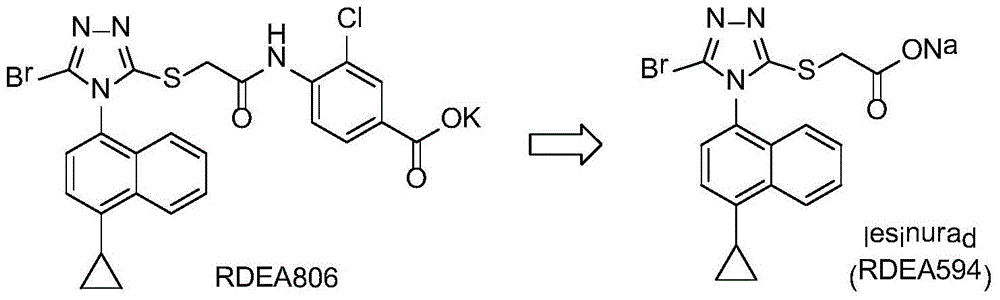
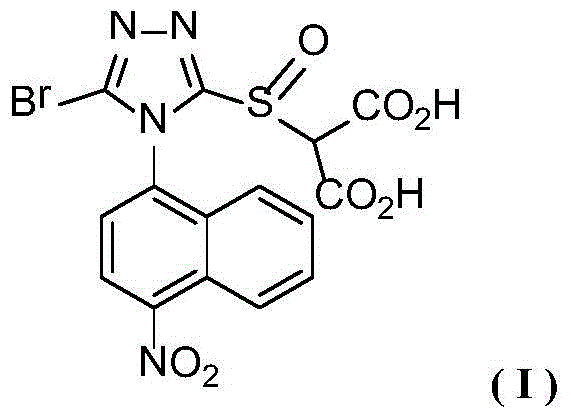
A nitro-substituted triazole sulfinyl malonate compound, its preparation method and use
A technology of dimethyl bromomalonate and compounds, applied in the field of uric acid transporter 1 inhibitors, can solve problems such as fulminant hepatitis, allopurinol liver and bone marrow toxicity, and allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The synthesis of embodiment 1 compound I
[0015]
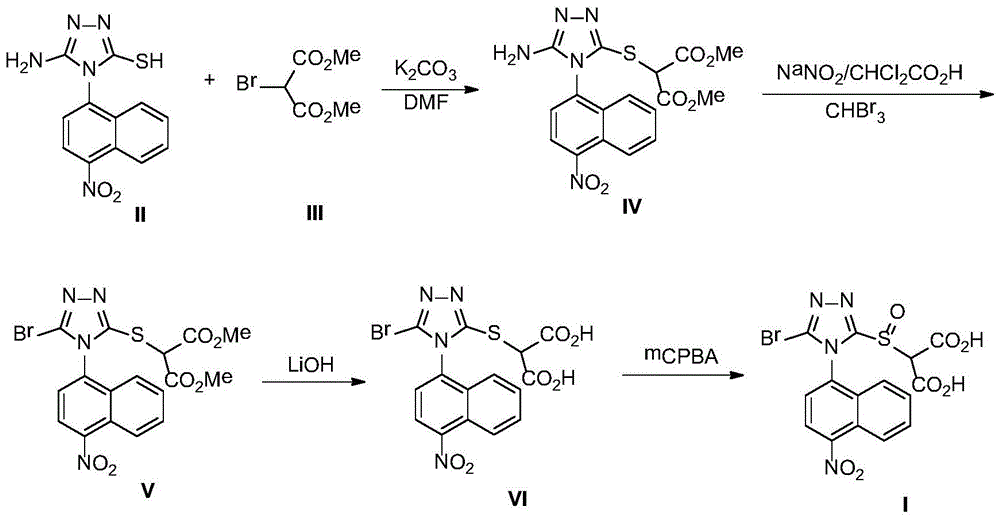
[0016] A. Synthesis of Compound IV
[0017] 5.74g (20mmol) of compound II and 4.22g (20mmol) of compound III were dissolved in 100mL of dry DMF, stirred at room temperature, and 8.29g (60mmol) of solid K was added 2 CO 3 , and then the reaction mixture was stirred at room temperature until TLC tracking found that the reaction was complete (generally within 12 h). The reaction mixture was poured into 400 mL of ice water, stirred, using 100 mL × 3 CH 2 Cl 2 After extraction, the extract phases were combined, washed with 100 mL of 5% brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IV, a white solid, ESI-MS, m / z=440 ([M+Na] + ).
[0018] B. Synthesis of Compound V
[0019] 5.00g (12mmol) compound IV was dissolved in 30mL ...
Embodiment 2
[0024] Embodiment 2 Preparation of comparative compound 1-2
[0025] In order to fully compare the beneficial effects of the compounds of the present invention, the present invention has prepared an undisclosed brand-new compound discovered by the applicant as a comparison, and the specific structure is as follows:
[0026] Comparative compound I-2
[0027] Its preparation method is as follows:
[0028]
[0029] A. Synthesis of Compound IV-2
[0030] 4.84g (20mmol) of compound II-2 and 4.22g (20mmol) of compound III-2 were dissolved in 100mL of dry DMF, stirred at room temperature, and 8.29g (60mmol) of solid K was added 2 CO 3 , and then the reaction mixture was stirred at room temperature until TLC tracking found that the reaction was complete (generally within 12 h). The reaction mixture was poured into 400 mL of ice water, stirred, using 100 mL × 3 CH 2 Cl 2 After extraction, the extract phases were combined, washed with 100 mL of 5% brine, and dried over anhy...
Embodiment 3
[0038] The compounds of the present invention and related compounds inhibit IC of URAT1 50 The values are determined in a similar manner as described in the literature (Example 12 in US2014 / 0005136).
[0039] Construction of a cell line stably expressing the humanized URAT1 transporter: The humanized URAT1 gene (SLC22A112) was subcloned from the plasmid pCMV6-XL-5 (Origene) into the eukaryotic expression plasmid pCMV6 / neo (Origene). Gene sequencing confirmed that the humanized URAT1 was consistent with the information recorded in the gene bank (NM_144585.2). HEK293 human embryonic kidney cells (ATCC#CRL-1573) were cultured in EMEM tissue culture medium under 5% CO 2 And cultured in 95% air atmosphere. pCMV6 / Neo / URAT1 was transfected onto HEK293 cells using L2000 type transfection agent (Invitrogene). After 24 hours, the transfected cells were divided into tissue culture dishes with a diameter of 10 cm, continued to grow for one day, and then the medium was replaced with f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com