Lentivirus used for preparing CART cells and having characteristics of efficient transfection capacity and biological activity
A wild-type virus and cell technology, applied in the field of lentivirus, can solve the problems of complex immunotherapy process, high cost and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158] Example 1: Construction of recombinant lentiviral vector
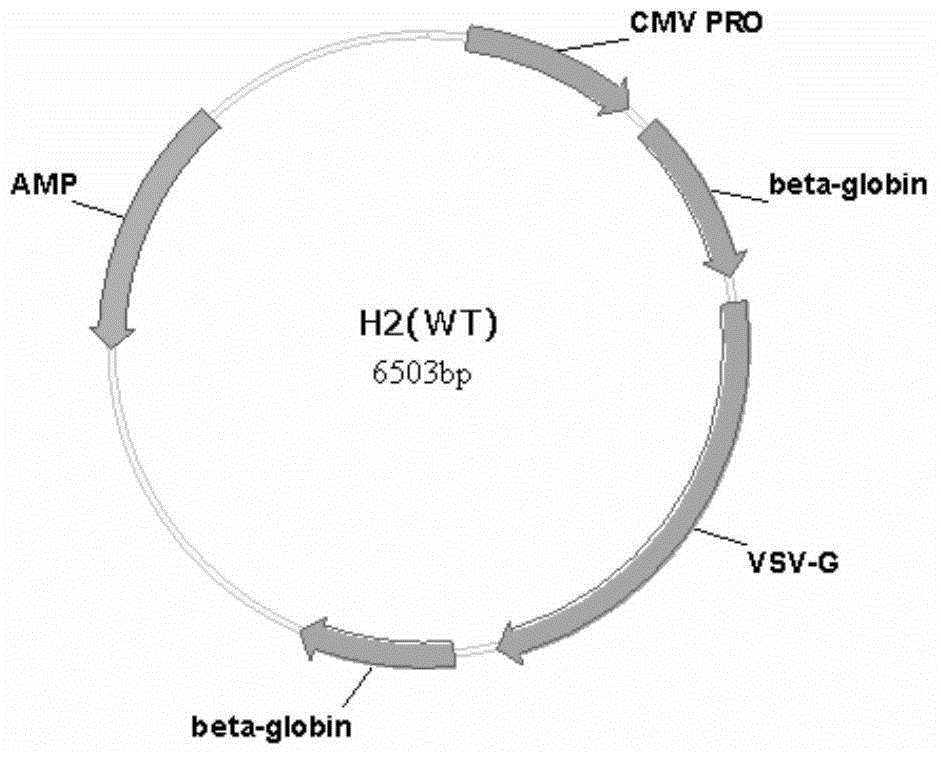
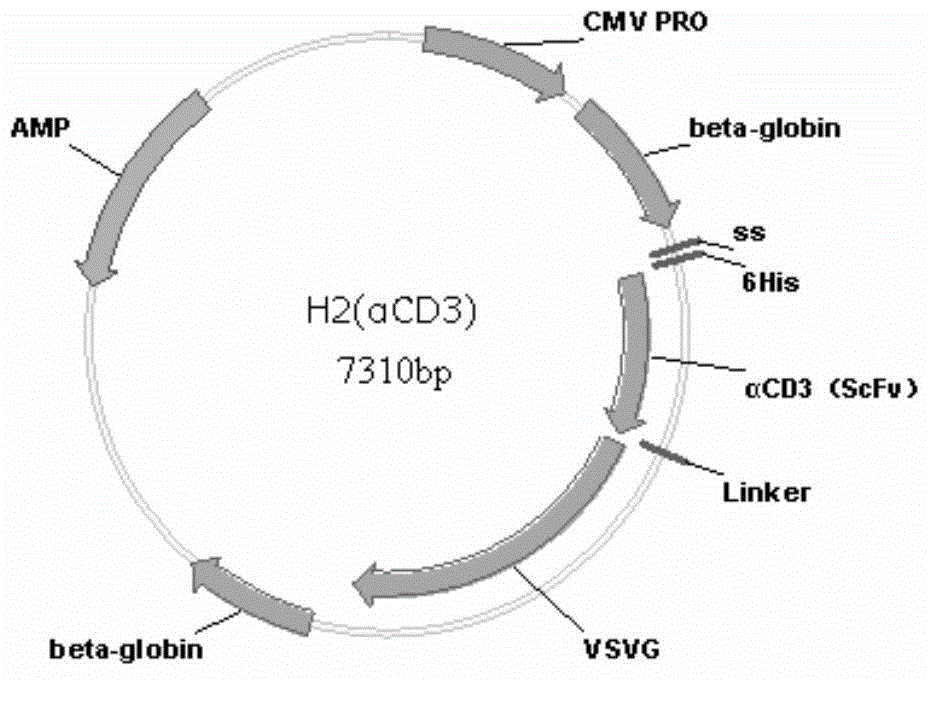
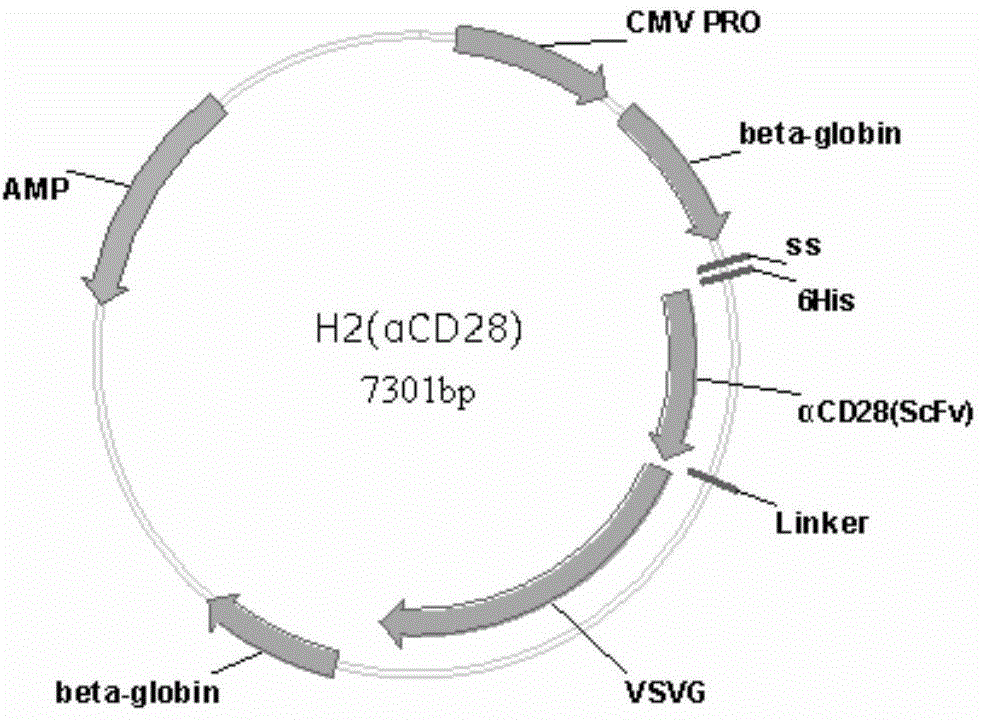
[0159] The schematic diagrams of the recombinant lentiviral vectors αCD3-VSVG and αCD28-VSVG have been described above.
[0160] The construction of the recombinant lentiviral vector is based on the viral packaging helper plasmid H2 (purchased from Addgene, namely pVSV-G) carrying the VSVG gene.
[0161] Construction of αCD3-VSVG recombinant lentiviral vector:
[0162] a) Obtaining the αCD3 gene fragment:
[0163] Whole gene synthesis signal peptide-6His-αCD3-flexible peptide fragment (the nucleotide sequence information of the signal peptide is SEQ ID NO.:8, the nucleotide sequence information of 6His is SEQ ID NO.:9, the nucleotide sequence information of αCD3 The nucleotide sequence information of the flexible peptide is SEQ ID NO.: 3, and the nucleotide sequence information of the flexible peptide is SEQ ID NO.: 10). Using the whole gene synthesis plasmid as a template, the αCD3 gene primer was designed, a...
Embodiment 2
[0209] Example 2: Recombinant lentivirus packaging and preliminary verification
[0210] 1. Supply HEK-293T cells (purchased from ATCC) according to the 24h passage cycle, change the medium before transfection, and use an electric pipette to replace 5ml of DMEM medium containing 2% FBS for each plate of cells.
[0211] 2. Add HBW buffer, plasmid H1 (purchased from Addgene, 12 μg / plate, plate refers to 10cm cell culture dish), recombinant H2 plasmid (10 μg / plate), vector plasmid (lentiviral vector containing exogenous genes) in sequence , purchased from Addgene, 24 μg / plate), CaCl2 (50 μl / plate), and finally 2×HBS (500 μl / plate) was added dropwise while shaking on a vortex shaker, and the transfection system was 1 ml / plate. The vector plasmid is a vector expressing EGFP initiated by EIF1α, and the recombinant H2 is a mixture of αCD3-VSVG, αCD28-VSVG and wild-type H2. Among them, αCD3-VSVG and αCD28-VSVG are added at a ratio of 1:1, and wild-type H2 is matched in different rati...
Embodiment 3
[0216] Example 3: Recombinant lentivirus infection of T lymphocytes
[0217] Infection experiments were performed according to conventional methods known to those skilled in the art. A brief description of the infection steps is as follows:
[0218] 1. Peripheral blood mononuclear lymphocytes (PBMC) were obtained, and >1x107 cells were obtained through the blood apheresis system.
[0219] 2. Use complete medium (TexMACS medium + 5% autologous serum) to adjust part of the cell suspension to make the final concentration 0.7 × 106 / ml, and add interleukin 2 (IL-2), penicillin (penicillin) and streptavidin streptomycin and bovine serum albumin (BSA), so that the final concentrations were 600IU / ml, 100U / ml, 100μg / ml and 0.2%.
[0220] 3. Gently mix the resuspended cells, take out 0.4ml of cell suspension (total cell volume 0.28×106) and transfer to a 1.5mL centrifuge tube.
[0221] 4. Add the required virus, and calculate the amount of virus added according to the MOI of 10.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com